OPTIFactor
Our Science | Fatigue | FAQ | Label Facts
OPTI Factor with the NT Factor® Nutrient Compound
 OPTI Factor is based on Membrane Lipid Replacement Therapy which has been shown to repair damaged cells.
OPTI Factor is based on Membrane Lipid Replacement Therapy which has been shown to repair damaged cells.
OPTI Factor™ is a custom formulation containing clinically studied amounts of NT Factor® with a mitochondrial fuel blend. Made up entirely of food and food extracts, it is just about the safest way to regain your youth. Opti Factor works by keeping your cells healthy, and fueling you’re mitochondria, the powerhouse of your cells. The ability to convert food and oxygen to energy begins in your cells. Taken in combination with other nutritional supplements, it makes everything work better.
Healthy Aging with Opti Factor™
- Restores Youthful Vibrancy
- Increases Your Energy Level by 40% or More
- Phosphoglycolipids
- Enhances and Restores Cellular Potential
WHY SHOULD I USE OPTI Factor?
- Renowned physicians have proven clinical studies demonstrating an increase in cellular energy.
- OPTI Factor™ does what vitamins can’t do . . . repairs damaged cells.
- Taking OPTI Factor creates sustained energy levels - not just a few hour jolt like energy drinks or caffeine. True, healthy energy!
Aging, stress, lifestyle, diet, medications, toxic environment and other factors weaken and damage our cells. OPTI Factor can repair and revitalize these damaged cells.
OPTI Factor™ with the NT Factor® nutrient compound has been clinically proven to reduce fatigue, increase energy and has been shown to cut biological age in half.
Click Here to Read Our Clinical Studies and Review Papers
Daily Vitamins Are Not Enough - We Need More
We take vitamins for nutritional support and antioxidant protection but no vitamin can protect all of our cells from becoming damaged. Damaged cells can lead us feeling tired at certain times of the day. In many cases more severe fatigue or exhaustion is experienced. OPTI Factor with the NT Factor® nutrient compound is the only scientifically proven product to repair damaged cells.
The NT Factor® nutrient compound has been shown to substantially eliminate fatigue and tiredness in pivotal studies conducted at UCLA, Henry Ford Hospital, The Institute for Molecular Medicine and others.
One key study showed biological findings of cellular health of 70 year old people equal to that of individuals 35 years of age.
One of the world’s leading experts in cellular research considered this to be one of the most important medical breakthroughs of the 21st century and defined this as “Lipid Replacement Therapy”.
What Do Researchers Say About The NT Factor® Compound Found in OPTI Factor?
According to Professor Garth Nicolson of the Institute for Molecular Medicine: “Aging, disease and degeneration place tremendous pressure on natural nutritional replacement processes. NT Factor® repairs damaged cells and restores their structure and function, thus it improves normal nutrient transport, communication and other functions to the states necessary for overall health and vitality”.
Here is an example of how OPTI Factor™ works to repair damaged cells
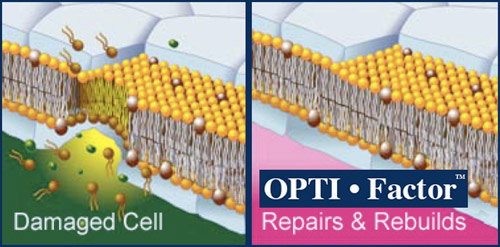 |
Anti-oxidants in our diet and supplements provide for protection against free radical damage to cell and mitochondrial membranes. But, what happens to the person who doesn’t take anti-oxidants until he/she is older, or is already experiencing chronic disease. It is widely accepted, in scientific circles, that free radical caused damage to membranes is the cause for chronic debilitating disease. For most people, when we are young our cells repair any free radical damage by readily producing enzymes, such as Superoxide Dismutase and proteins such as glutathione that serve as anti-oxidants. For a variety of reasons including diet, stress and other causes our cells begin to lose the ability, slowly at first, to stop a free radical from damaging it’s membrane.
The first event in the development of chronic disease is free radical damage to a cell membrane that is not immediately repaired. The unrepaired damage weakens the cell, and so, allows more free radical damage. The accumulation of the free radical damage causes the membrane to become dehydrated and rancid. Anti-oxidants from the food supply do not have the capability to significantly repair damaged membrane. Anti-oxidants normally formed in the body for the purpose of repairing membranes depend on a healthy membrane in order to be produced. Once damage to membranes begins it becomes a process that increases in frequency and accumulation. It gives a logic to the cliché, ‘She aged overnight!”. Now there is an opportunity to slow that process down dramatically.
OUR SCIENCE
OPTI Factor and Mitochondrial Function
OPTI Factor contains NT Factor® which is the most powerful proprietary substance to combat aging and fatigue.
by MICHAEL D. SEIDMAN, MD.
A study to investigate the effects of polyunsaturated phosphatidyl-choline (PPC)a on aging — in general, as well as the particular matter of age-associated hearing loss, was conducted. Regarding the latter, this report supports that the Membrane Hypothesis of Aging (MHA, also known as the Mitochondrial Clock Theory of Aging) provides a plausible explanation for age-related hearing loss. According to this hypothesis, Reactive Oxygen Metabolites (ROM) are responsible for progressive insults on mitochondria and other cellular structures. Over an extended time period, these insults accumulate leading to a reduction in the energy generating capacity of the mitochondria, cellular demise and resultant senescence.
Soy lecithin is a source from which PPC can be extracted. PPC molecules are indispensable for cellular differentiation, proliferation and regeneration. High-energy functional and structural elements of all biological membranes, PPC plays a rate-limiting role in the activation of numerous membrane-located enzymes, including superoxide-dismutase and glutathione peroxidase, which are important antioxidants that protect cell membranes from damage by reactive active metabolites (ROM).
PPC are highly purified extracts of the semen of soybean, supplying the organism with nontoxic choline molecules with a high content in polyunsaturated fatty acids — in particular, linoleic acid. These PPC correspond to the body’s own PPC molecule. The physiologic functions of these phospholipids are related to the morphology of the biological membranes, the incorporation of these molecules into membranes and thus on the intact character of the structure of cell membranes.
There are several disease processes related to membrane damage for which clinical and pharmacological trials using PPC have been conducted. Effects of PPC on these various disorders have shown enhancement in cognitive performance of the aging brain, improvement of coronary, peripheral and cerebral blood flow, activation of liver metabolism and detoxification and promotion of gastrointestinal function by mucosal restoration. The current study was designed to investigate the effects of PPC on age-related hearing loss by evaluating its ability to preserve mitochondrial function, protect mitochondrial DNA from oxidative damage and preserve auditory sensitivity.
Harlan-Fischer 344 rats, 18-20 months of age, were used as the experimental subjects. The subjects were caged individually and maintained at 21 to 22° C in a 12:12 light-dark cycle.b A dose of 300mg/kg/day of NT Factorc was supplemented to each subject, by adding it to the oral diet. The animals were divided randomly into two groups (n = 7 for each group). Group-1 served as the control, and group-2 as the experimental group. At the onset of the study, Auditory Brainstem Responses were obtained to measure base-line hearing thresholds in all subjects. Age-associated changes in hearing sensitivities were then recorded at two-month intervals for six months. In order to assess age-related changes in mitochondrial function, mitochondrial membrane potentials were studied using flow cytometry. For this purpose, peripheral blood was obtained from each subject at the beginning and at the end of the protocol. At the conclusion, the subjects were euthanized (according to NIH protocol), and tissue samples were obtained from brain and cochlea (stria vascularis and auditory nerve) to study mitochondrial DNA deletion associated with aging. Ihis was achieved by amplifying the specific common aging mitochondrial deletion (4834-bp) by Polymerase Chain Reaction. DNA quantification was performed. The data obtained for each protocol was compared between the two groups and analyzed using ANOVA.
The effects of PPC on age-related hearing loss demonstrate a gradual age-associated decline in hearing sensitivities at all the frequencies tested (3, 6, 9, 12 and 18 kHz). These results are comparable to previous studies that have shown similar results under similar experimental conditions. There was a statistically significant preservation of hearing noted in the treated subjects at all frequencies, which was observed at four and six months of treatment. Overall, there was a continued decline in hearing in the control subjects and a statistically significant protective effect of PPC on the experimental subjects (p<.005).
Mitochondrial membrane poten-tials were recorded by flow cytometry as a measure of the uptake of Rhodamine 123 by mitochondria. This probe is specific for mitochondria as it is selectively taken up by the mitochondrial membrane. The intensity of this uptake corresponds directly to the mitochondrial activity and hence membrane potential. The data obtained from the two groups were averaged and statistical analysis was performed using ANOVA. The mean fluorescence intensity (MFI) in group-1 subjects measured 3190 and 2100 at the beginning and end of the study, respectively. Ths, approximately, 30% decline in membrane potential with time was statistically significant (p=O.OO3). Con-versely, the MFI in the experimental group remained essentially unchanged at 2990 from 3165 at the beginning of the study. This difference between the control and treated groups was statistically significant (p<O.O5), demonstrating the protective effect of PPC supplementation on mitochondrial membrane potential.
For the mtDNA deletion tests, mtDNA from brain, stria vascularis and auditory nerve were studied. In order to verify the presence of mtDNA, the ND-1 16S rRNA segment was identified, which is a highly preserved region of the mitochondrial genome. Specific primers for this segment and for the common aging deletion were synthesized in our laboratory. Equal quantities of DNA were used in all samples for standardization purposes. The PCR products identified the ND-1 16SrRNA region (a control to verify the presence of mitochondrial DNA) by a 6O1bp product in all samples and the common aging deletion (4834 bp deletion) by a 598 bp product. This aging deletion was identified in five of the seven control subjects and four of the experimental subjects. Quantitative deter-mination reveals a significantly lower ratio of this common aging deletion to the total mtDNA in the experimental subjects as compared to the control subjects. Based upon these findings we conclude that PPC has a protective effect on mitochondrial DNA damage and function.
Reactive Oxygen metabolites (ROM) are known to play important roles in many biochemical reactions that are critical in maintaining normal cell functions. Increasing evidence indicates that ROM are also important mediators of several forms of tissue damage, such as injuries associated with inflammatory responses, ischemic injuries to tissues, injuries resulting from the intracellular metabolism of chemicals and drugs, coronary artery disease, cerebrovascular accidents, age-related hearing loss and aging. The primary in vivo source of ROM appears to be the mitochondrial electron transport system during oxidative phosphorylation (during the process of energy generation). There are many other sources of ROM production including; prostaglandin biosynthesis, environmental contaminants, cigarette smoking, ionizing radiation and poor dietary regimens.
ROM generation occurs from periods of prolonged relative hypo perfusion, such as can be seen with arteriosclerosis and aging. It has been demonstrated that in the elderly population there is significantly decreased flow within the circulatory system in general,1-5 and the inner ear, in specific.6-9 Prolonged periods of reduced blood flow such as those accompanying aging lead to the formation of tissue damaging ROM. ROM have been implicated in injury to polyunsaturated fatty acids in cell membranes resulting in the process of auto-oxidation which is of great importance in the pathogenesis of cell membrane damage. They have also been shown to be mediators of mitochondrial DNA damage including the generation of mitochondrial DNA deletions (mtDNA del). MtDNA del have been associated with cellular and tissue dysfunction, age-related hearing loss,19 senescence and death. This sequence of events is the foundation of the membrane hypothesis of aging (MHA)8-10
Phospholipids are integral structural components of all biological membranes with PPC and phosphotidylethanolamine being the predominant types, quantitatively. They constitute the phospholipid bilayer structure of cellular membranes, which is responsible for membrane stability and cellular function. PPCs maintain and promote the activity of several membrane bound proteins and enzymes, including Na-K ATPase, adenylate cyclase and glutathione reductase. They are also known to be precursors of cytoprotective agents such as eicosanoids, prostaglandins and antioxidants.
These experiments suggest that NT Factor containing PPC may protect mitochondrial function by preserving the age-related decline in mitochondrial membrane potentials and hence their activity. Additionally, there was less mitochondrial DNA damage noted in the treated group. This may also explain the demonstrated effect of preservation of hearing loss associated with aging, by the ability of PPC to specifically up regulate cochlear mitochondrial function. There are many studies demonstrating the effects of mitochondrial metabolites on cognition and aging,11-18, 20-22 Additionally, recent work from our laboratory has shown that acetyl-Lcarnitine and a-lipoic acid delay the progression of age-related hearing loss by protecting cochlear mitochondrial DNA from oxidative damage.23 These results support the membrane hypothesis of aging and provide further evidence to support this theory as a possible explanation for age-related hearing loss. Thus, PPC may be one of many rational approaches to consider for the purpose of membrane preservation, enhanced mitochondrial function, reduction of age-associated mitochondrial DNA damage and slowing of some of the aging processes.
FOOTNOTES
A. NT Factor™ is a registered trademark of Nutritional Therapeutics Inc. Smithtown, NY, USA. NT Factor is comprised of defatted rice bran, arginine, beet root fiber, black strap molasses, glycine, magnesium sulfate, polyunsaturated phosphatidylcholine (phospholipids), saponin (glycolipids), para-amino benzoate, leek, pantethine (bifidus growth factor), taurine, garlic, calcium borogluconate, omega-6 essential fatty acids, omega-3 essential fatty acids, artichoke, barley malt, potassium citrate, calcium sulfate, spirulina, bromelain, natural vitamin E, calcium ascorabte, alpha-lipoic acid, oligosaccharides, B-6, niacinamide, riboflavin, inositol, niacin, calcium pantothenate, thiamin, B-l2, bifidus, acidophilus, folic acid, chromium picolinate.
B. The experimental protocols were reviewed and approved by the Care for Experimental Animal Committee (CEAC) at the Henry Ford Health System. These protocols are in strict compliance with guidelines as established by the National Institute of Health.
C. NT Factor containing PPC procured from Nutritional Therapeutics, Smithtown, NY, USA.
REFERENCES
1. Gates GA, Caspery DM, Clark Wet al. Presbyacusis. Otolaryngol Head & and Neck Surg 1986; 100: 266-271
2. Kimura RS, Schuknecht HF. The ultra structure of the human stria vascularis. Acta otolaryngologica. 1970; 69(6): 415-427
3. Harkins SW. Effects of age and interstimulus interval on the brain stem auditory evoked potential. Inter.J. Neuroscience. 1981; 15(1-2):107-118
4. Rosenhall U, Pederson K, Dotevall M. Effects of presbycusis and other types of hearing loss on auditory brain stem responses. Scand. Audiol. 1986; 15 (4): 179-185
5. Hoeffding V, Feldman ML. Changes with age in the morphology of the cochlear nerve in rats: light microscopy. J. comparitive neurology 1988; 276(4):537-546
6. Axelsson A. The cochlear blood vessels in guinea pigs of different ages. Acta Otolaryngol. (Stockh.) 1971; sept. 72 (3): 172-181
7. Seidman MD, Khan MJ, Dolan D, Quirk WS. Age-related differences in cochlear microcirculation and auditory brain stem response. Arch. Oto Head & Neck Surg 1996; 122: 122 1-1226
8. Wallace DC. Mitochondrial genetics: a paradigm for aging and degenerative diseases? Science. 1992 May 1; 2 56(5057): 628-32
9. Seidman MD, Bai U, Khan MJ et al. Association of Mitochondrial DNA deletions and cochlear pathology: A molecular biologic tool. Laryngoscope. 1996; 106: 777-783 10
10. Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc. Natl. Acad, Sci. USA. 1994; 91:1077 1-10778
11. lmperato A, Ramacci TM, Angelucci L. Acetyl L-carnitine enhances acetylcholine release in the striatum and hippocampus of awake freely moving rats. Neuroscience letters. 1989: 107(1-3): 251-255
12. Ghirardi 0, Milano S, Ramacci MT, Angelucci L. Effects of acetyl L-carnitine chronic treatment on discrimination models in aged rats. Physiol. Behav. 1988;44(6): 769-773
13. Caprioli A, Ghirardi 0, Ramacci MT, Angelucci L. Age-dependent deficits in radial maze performance in the rat: effect of chronic treatment with acetyl Lcarnitine. Prog. in Neuropsychopharmacol. Biol. Psychiatr. 1990; 14(3): 3 59-369
14. Bast A and Haenen GRMM. Interlay between lipoic acid and glutathione in the protection against microsomal lipid peroxidation. Biochem. Biophys. Acta. 1988; 963: 558-561
15. Suzuki YJ, Aggarwal B, Packer L. Aipha-lipoic acid is a potent inhibitor of NFKb activation in human T cells. Biochem. Biophys. Res. Commun. 1992; 189:1709-17 15
16. Kagan VE, Shvedova A, Serbinova E, Khan S, Swanson C, Poweel R, Packer L. Dihydrolipoic acid: A universal antioxidant both in the membrane and in the aqueous phase. Biochem Pharmacol. 1992; 44: 163 7-1649
17. Devasagayam TP, Subramanian M, Pradhan DS, Sies H. Chemical Biological Interactions. 1993; 86: 79-92
18. Gadaleta MN, Petruzalla V, Daddabbo L, et al. Mitochondrial DNA transcription and translation in aged rat. Effect of acetyl-L-carnitine. Ann.N.Y. Acad. Sci. 1994; 717: 150-160
19. Bai U, Seidman MD, Hinojosa R, Quirk WS. Mitochondrial DNA deletions associated with aging and possibly presbyacusis: A Human archival temporal bone study. Am.J. Otology. 1997; 18(4): 1-5
20. Paradies G, Ruggiero FM, Petrosillo G, Gadaleta MN, Quaglieriello E. Carnitine-acylcarnitine translocase activity in cardiac mitochondria from aged rats: the effect of acetyl-L-carnitine. Mech. of aging and develop. 1995; 84(2):103-1 12
21. Aureli T, Miccheli A, Ricciolini R, Di Cocco M, et al. Aging brain: effect of acetyl L-carnitine treatment on rat brain energy and phospholipid metabolism. A study by 3 1P and 1H NMR spectroscopy. Brain Research. 1990; 526(1):108-1 12
22. Sebinova E, Khwaja S, Reznick AZ, Packer L. Thioctic acid protects against ischemia-reperfusion injury in the isolated perfused Langendorif heart. Free Rad. Res. Commun. 1994; 17: 49-58
23. Seidman MD, Khan MJ, Bai U, Shirwany N, Quirk WS. Biologic Activity of Mitochondrial Metabolites on Aging and Age-Related Hearing Loss. Am. J. Otol. 2000;21:161-167.
Michael D. Seidman, MD., FACS, is a member of the Department of Otolaryngology Head and Neck Surgery at Henry Ford Health System, West Bloomfield, Michigan, USA
OPTI Factor and Mitochondrial Function and Supplements
OPTI Factor contains NT Factor® which is the most powerful proprietary substance to combat aging and fatigue.
Published in the Townsend Letter for Doctors and Patients July, 2003
Chronic Fatigue, Aging, Mitochondrial Function and Nutritional Supplements
Garth L. Nicolson, Ph.D.1,2
1 Professor of Molecular Pathology, The Institute for Molecular Medicine, Huntington Beach, CA
2 Professor of Integrative Medicine, Capital University of Integrative Medicine, Washington, DC
Abstract
Intractable fatigue is the most common complaint of patients seeking medical care, and in most patients it is a chronic condition that is not reversed by sleep or rest. Although fatigue is a complex phenomenon, it has been defined recently as a multi-component sensation. It is related to aging, decreased mitochondrial function and loss in the ability of mitochondria in cells to produce high-energy molecules for cellular functions. Also, it is known that oxidative damage to mitochondria, mainly from Reactive Oxygen Species or ROS, resulting in modifications in mitochondrial lipids, proteins and DNA, is related to aging. Certain natural dietary products and supplements can reduce oxidative damage and replace high energy molecules or restore mitochondrial function. Recent clinical trials have shown the benefit of dietary supplements in restoring mitochondrial function and reducing fatigue. In aging subjects mitochondrial function was restored to levels found in young adults in consort with reductions in fatigue, suggesting the anti-aging and anti-fatigue benefits of protecting mitochondria and cells from oxidative and other molecular damage by lipid replacement and antioxidant use
Introduction-What is Fatigue?
The most common complaint of patients seeking medical care from general medical practitioners is fatigue or loss of energy, and in fact, chronic fatigue (intractable fatigue lasting more than 6 months and not reversed by sleep) is reported by approximately one quarter of all patients seeking medical care.1,2 Many medical conditions are associated with chronic fatigue, such as respiratory, coronary, skeletal-muscular and bowel conditions as well as various cancers and infections,3,4 and chronic fatigue is often an important secondary condition in many clinical diagnoses. Loss of energy and the symptom of fatigue often precede and are usually related to clinical diagnoses, and this may be the most important reason that it is so commonly reported by patients seeking medical care.5
Fatigue has been in the medical literature for hundreds of years in many forms and indicated by several different historical terms, but it has been only recently that fatigue has been defined and attempts made to determine the extent of fatigue and its possible causes. Although we now know much more about fatigue, its universal definition remains to be determined. It is thought to be a multidimensional sensation with many possible causes.1,2 Most patients understand fatigue as a loss of energy and inability to perform even simple tasks without exertion.
Recently Piper et al.4 described fatigue as a multi-component sensation with behavioral (interference with normal activities), affective (how fatigue is described), sensory (feelings associated with fatigue) and cognitive (mood, memory and thinking) components. They also designed a simple measurement tool for assessing fatigue that combined multiple fatigue-associated elements into an overall fatigue score.4,5 We have successfully used this validated instrument in clinical studies on aging subjects to determine their fatigue responses to various dietary supplements.6,7
Fatigue at the Cellular Level-Role of Mitochondria
At the cellular level fatigue is involved with cellular energy systems that for the most part are found in the mitochondria. Mitochondria are specialized semi-autonomous cellular organelles with their own lipid membranes, enzymes and DNA genetic information, and they degrade and convert sugars and lipids to energy that is stored in high-energy molecules (ATP, NADH, etc.) using oxygen and a system called the mitochondrial electron transport chain. The electron transport chain is responsible for oxidative phosphorylation, the principal source of high-energy molecules in every cell. Although mitochondria appear to be semi-autonomous, separate units within our cells; in fact, they are completely dependent functionally on many proteins and enzymes that are made by other parts of the cell and encoded by nuclear DNA.
Without the proper functioning of mitochondria, our cells must depend on anaerobic sources of metabolism to produce high-energy molecules from starches and sugars, resulting in the production of lactic acid as a byproduct of sugar metabolism. Everyone at one time or another has noticed what happens when we over-exert physically and cannot provide enough oxygen for our mitochondria, and our cells must resort to sources such as anaerobic metabolism to produce high-energy molecules such as ATP for our muscles. Eventually our muscles cramp due to the build-up of lactic acid and other metabolites. Thus our mitochondria are our most important sources of high-energy molecules for building and maintaining cellular functions in an oxygen environment.
Oxidative Damage to Mitochondria and Aging
Damage to cellular mitochondria can impair the abilities of cells to produce high-energy molecules, and this occurs naturally with aging, mainly by the build up of oxidative damage to mitochondrial molecules. During aging the production of Reactive Oxygen Species or ROS, made up of oxidative and free radical molecules, such as nitric oxide, oxygen and hydroxide radicals and other oxidative molecules, can cause oxidative stress and cellular damage, resulting in oxidation of lipids, proteins (enzymes) and DNA in cells. Once oxidized, these cellular molecules can be deactivated or structurally and functionally changed. Major targets of cellular ROS damage are mitochondria and nuclei, mainly their phospholipid/protein membranes and DNA,8-11 resulting in damage to membrane lipids and protein enzymes and deletion or modification of DNA.
ROS production and damage to mitochondria and nuclei occur throughout our lifetimes, but we have natural cellular systems that neutralize excess ROS and repair ROS-mediated damage. Although some ROS production is actually important in triggering cell proliferation and gene expression, with aging ROS damage accumulates. For example, cellular antioxidant enzymes normally neutralize excess ROS and enzyme repair mechanisms, or biosynthesis systems restore ROS-damaged molecules or replace them. However, when the concentration of ROS far exceeds the ability of cells to neutralize ROS or repair or replace ROS-mediated alterations, molecular damage accumulates within cells. Typically this occurs in aged animals and humans, but disease and infection can also result in similar damage that exceeds the abilities of cellular systems to neutralize, repair or replace damaged molecules.
In contrast to mitochondria isolated from young animals, mitochondria from aging animals show higher levels of accumulated ROS damage to mitochondrial membranes, enzymes and DNA.12 At the molecular level, damage to phospholipids and other lipids in mitochondrial membranes by ROS free-radicals can affect membrane integrity, membrane fluidity and transmembrane electrical potentials, resulting in loss of energy production by the electron transport chain and its associated components. This occurs because the functional status of the mitochondrial electron transport chain is dependent on the integrity of mitochondrial membranes and maintenance of an electrical potential across the membranes.
Young cells and young organisms can cope with ROS since they possess high levels of free-radical scavenging systems that neutralize ROS, such as the enzymes superoxide dismutase and glutathione reductase. They also have a high capacity to repair or replace damage caused by ROS. With aging this system can decline or be overwhelmed by ROS and oxidative stress.12,13 Since the aging process results in mitochondria suffering accumulated ROS damage to their membranes and DNA, this is thought to contribute to or even be a cause of the aging process.9,12,13 It is also important in fatigue, as will be shown below. In animals caloric restriction has been used to extend longevity, and this also reduces oxidative stress and oxidative damage to tissue mitochondria.14
Reducing ROS-mediated Damage
Reducing cellular and mitochondrial membrane and DNA damage and loss of membrane integrity are important in preventing loss of cellular energy and regulating cellular life span.15 This can be done by neutralizing ROS with various antioxidants or increasing free-radical scavenging systems that neutralize ROS. Some common dietary antioxidants are shown in Table 1 along with some accessory molecules that are important in maintaining free-radical scavenging systems, biosynthetic capacity, immune systems and other important cellular functions. Although this list is incomplete, the antioxidants and accessory substances shown in Table 1 have been commonly used as anti-aging supplements as well as substances to help prevent or lessen the effects of various chronic and degenerative diseases. There are at least 40 micronutrients required in the human diet,16 and aging increases the need to supplement these in a normal diet to prevent age-associated declines in mitochondrial and other cellular functions.
In animal studies the effects of reducing ROS have been dramatic in aging and disease models. For example, in rodents there are age-dependent losses in antioxidants, such as vitamins C and E, as well as reductions in reduced glutathione and the levels of antioxidant enzymes.16,17 Using aged rats the effects of alpha-lipoic acid and other dietary antioxidants on the levels of cellular antioxidants, such as reduced glutathione and vitamins C and E, levels of mitochondrial membrane lipid peroxidation and activities of mitochondrial electron transport and accessory enzymes were investigated.18 Supplementation with antioxidants reduced mitochondrial lipid peroxidation, decreased levels of ROS and increased amounts or activities of certain electron transport enzymes. These authors found that dietary antioxidant supplementation reversed the age-related declines in cellular antioxidants and mitochondrial enzyme activities and prevented mitochondria from age-associated functional decline.
In another study rats were fed diets supplemented with coenzyme Q10, alpha-lipoic acid, melatonin or alpha-tocopherol for a six-month period. They found that antioxidants could inhibit the progression of certain age-associated changes in cerebral mitochondrial electron transport chain enzyme activities.19 Similar results in rats using dietary coenzyme Q10 and other antioxidants were found in Japan.20 Thus animal studies have shown that antioxidants can prevent the aging-associated changes in mitochondrial structure and function.
In addition to the aging-associated oxidative changes in mitochondrial enzymes and lipids, mitochondrial DNA also accumulates oxidative damage during the aging process.12,13,21 To prevent this antioxidants have also been useful, such as vitamins C and E, coenzyme Q10, sulfur-containing antioxidants and plant antioxidant extracts.22,23 Age-associated damage to mitochondrial DNA may affect their ability to function due to a loss in the ability to synthesize and replace critical mitochondrial enzymes.
Antioxidants may also affect the pathogenic processes of certain diseases. In a mouse model for Amyotrophic Lateral Sclerosis (ALS) or Lou Gehrig’s Disease, a neurodegenerative disease that results in brain motorneuron death, dietary coenzyme Q10 significantly increased lifespan and provided some neuroprotective effects, including decreased loss of nerve mitochondria.24 The experimental dietary use of antioxidants can prevent age-associated mitochondrial dysfunction and damage, inhibit the age-associated decline in immune function and prolong the lifespan of laboratory animals.25
Clinical Studies on Antioxidants
There are few clinical studies, unfortunately, that have used the information from animal research to investigate the role of multiple dietary antioxidants in human aging and disease. Of course, one of the problems facing researchers who conduct clinical trials is the widespread use of vitamins and antioxidants by the general population that could affect such trials. Although the results obtained from controlled animal studies are backed up by studies in vitro using cultured human cells,26 there have been only a few clinical trials that directly address the role of antioxidants in preventing mitochondrial damage during aging and disease. Major problems in designing and conducting such trials are that it is extremely unlikely that a single or even a few antioxidants can produce significant effects and prevent aging-associated changes or affect pathogenic processes and the problem that each individual may have optimum levels of antioxidants that could be suboptimal for others. Also, the number of various different antioxidant combinations and concentrations that could be used in controlled clinical trials is daunting.
Nonetheless, there have been clinical trials that have found some interesting results. One of the few well controlled clinical studies on antioxidants examined their role in preventing ultraviolet (UV) damage to skin cells in 100 young and aged healthy subjects.27 Damage was measured by the UV-induced accumulation of oxidized lipids and reductions in natural antioxidants, such as vitamin E and coenzyme Q10. They found age-associated increases in oxidized lipids and decreases in natural antioxidants, and UV irradiation worsened these in a dose-dependent manner but this could be prevented, in part, by increasing antioxidant concentrations through dietary intervention.
Clinical research has just begun to examine the use of combinations of antioxidants in dietary supplements in reducing increased oxidative stress found in aging. For example, in one study various formulas containing mixtures of dietary antioxidants were studied for their effects on oxidative stress using a method that detects metabolic derivatives of ROS action. Using healthy volunteers they compared the effects of low-dose combinations of (1) zinc, selenium, vitamin A (as retinol acetate), beta-carotene, vitamin E (as alpha-tocopheryl acetate) and L-cysteine, (2) citrus bioflavonoids, vitamin C (as L-ascorbic acid), coenzyme Q10 and vitamin B-6 (as pyridoxine hydrochloride) and (3) a combination of dietary formulations 1 and 2. The formulations were administered in a cross-over study where subjects received placebo and then test samples or the converse. They found that formulations 1 and 3 significantly reduced ROS metabolic derivatives in most of the subjects but formulation 2 did not. Future studies will have to expand the list of potential antioxidants and determine more optimal doses of antioxidants for dietary use, but it may be necessary to individualize such formulations to reach optimal antioxidant combinations in each individual.
Clinical Studies on High-Energy Molecules
Another method to increase the concentrations of high-energy molecules used by cells, such as ATP and NADH, is to administer these in dietary formulations. Unfortunately, this cannot be easily done with the very unstable, high energy phosphorylating molecule ATP, but it can be done with reduced NAD or NADH which can be converted inside cells to ATP. Although NADH can be administered in a dietary formulation, it is very unlikely that this alone is sufficient to reach cells intact at effective concentrations after oral administration. The reason for this is that NADH is quickly converted to low-energy forms in the gut and during transport in the blood.
To prevent breakdown of NADH a stabilized oral form that can be absorbed by the gut without degradation has been devised called ENADATM (www.enada.com). This form was used to assess the effects of NADH on 26 Chronic Fatigue Syndrome patients in a plabeco-controlled clinical trial of cross-over design where patients receive placebo or test samples for four weeks, then switch to one or the other midway during the trial for another four weeks after a four week wash-out period. In this trial 8 of 26 (31%) patients responded favorably to NADH in contrast to 2 of 26 (8%) to placebo. Response was measured by improvements in signs and symptoms reported by patients.28 In a follow-up pilot study these same authors report that 72% of patients who used ENADATM experienced some improvement in clinical signs and symptoms associated with fatigue. Unfortunately, these clinical trials did not use a validated fatigue assessment instrument to determine the effects of ENADA on fatigue, so it is difficult to actually determine how effective the product is suppressing fatigue.
Animal Studies using Lipid Replacement Therapy
Another method that has been used to reverse damage to tissue mitochondria is to replace damaged mitochondrial membrane phospholipids and other lipids by replacement therapy. This has been accomplished by replacement of damaged lipids using a dietary supplement containing polyunsaturated phosphatidylcholines and other phospholipids and fatty acids that are essential structural and functional components of all biological membranes.6,7 This dietary supplement is called NTFactorTM (www.NTFactor.com), and it has been used successfully in animal and clinical lipid replacement studies because the encapsulated lipids are protected from oxidation and can be picked-up and transported into tissue cells without undue oxidation.6,7 NTFactorTM contains a variety of components, including glycolipids and other lipids, nutrients, probiotics, vitamins, minerals and plant extracts (Table 2).
Using NTFactor an anti-aging effect has been demonstrated in aging rats. In 18-20 month-old rats Seidman et al.29 found that NTFactor prevented hearing loss associated with aging, shifting the threshold hearing from 35-40 dB in control aged animals to 13-17 dB in the test group. These results were significant (p<0.005). They also found that NTFactor preserved cochlear mitochondrial function as measured in a Rhodamine-123 transport assay, increasing mitochondrial function by 34%. (Rhodamine-123 is transported into mitochondria where it is reduced only under conditions where mitochondria are fully functional)30 NTFactor also prevented the common aging-related mitochondrial DNA deletion (mtDNA4834) found in the cochlear of aging rats.29 Thus lipid replacement in an animal model of aging was successful in preventing age-associated hearing loss and mitochondrial damage.
Clinical Studies using Lipid Replacement Therapy
Lipid replacement therapy has been successfully used in clinical studies to reduce fatigue and protect cellular and mitochondrial membranes from damage by ROS. For example, NTFactor has been used in a vitamin and mineral mixture (PropaxTM; www.propax.com) in cancer patients to reduce the effects of cancer therapy, such as chemotherapy-induced fatigue, nausea, vomiting and other side effects associated with chemotherapy.31
In a twelve week double-blinded, cross-over, placebo controlled, randomized trial on cancer patients receiving chemotherapy PropaxTM supplementation resulted in improvement from fatigue, nausea, diarrhea, impaired taste, constipation, insomnia and other quality of life indicators.31 Most (64%) of the patients in the study reported significant improvement in these and other chemotherapy-induced side effects, and 29% experienced no overall worsening of side-effects. Following cross-over to the supplement containing patients receiving the Propax supplement reported rapid improvement in nausea, impaired taste, tiredness, appetite, sick feeling and other indicators.
We have used Propax plus NTFactor in a pilot study with severely fatigued, aged subjects (>60 years-old) with a variety of clinical diagnoses to reduce fatigue, as measured by the Piper Fatigue Scale.4,5 We found that fatigue was reduced approximately 40%, from severe to moderate fatigue, after eight weeks of using Propax containing NTFactor. The results were highly significant (p<0.0001).7
A more recent study was initiated to examine the effects of NTFactor on fatigue in moderately and mildly fatigued subjects and to determine if their mitochondrial function, as measured by the transport and reduction of Rhodamine-123,30 improved with administration of NTFactor in concert with improvements in fatigue scores. The results of this clinical trial are shown in Figure 1.6 After eight or twelve weeks of NTFactor, there was a 33% or 35.5% reduction in fatigue, respectively. The results were highly significant (p<0.001) and were obtained using a validated instrument for measuring fatigue.
In the lipid replacement trial with moderately fatigued patients reductions in fatigue paralleled the significant gains in mitochondrial function.6 In fact, there was good correspondence between fatigue and mitochondrial function (Figure 1).6 Mitochondrial function was significantly (p<0.001) improved by the use of NTFactor for eight weeks. Interestingly, after 12 weeks of NTFactor use mitochondrial function was found to be similar to that found in young, healthy adults (Figure 1).6 After 12 weeks of NTFactor, subjects discontinued the supplement for 12 weeks and their fatigue and mitochondrial function were then measured. Their fatigue and mitochondrial function were intermediate between the starting values and those found on eight or 12 weeks of NTFactor, indicating that continued use of the supplement is likely required to maintain lower fatigue scores and show improvements in mitochondrial function. The results indicate that mitochondrial lipid replacement therapy can significantly restore mitochondrial function and improve fatigue scores in aging human subjects.
Mitochondrial Function, Fatigue and Degenerative Disease
Mitochondria are the most important source of cellular energy in our bodies. If their function is impaired, energy available to cells is limited to the Krebs Cycle. There are a number of conditions and substances that can impair mitochondrial function,8-10 but oxidation and damage of mitochodrial lipids in membranes are among the most important causes of impairment of mitochondrial function.32 This can result in modification of the electrical potential barrier across the mitochondrial membranes that is essential in the electron transport chain generation of cellular high-energy molecules.32 Mitochondrial function appears to be directly related to fatigue, and as patients experience fatigue their mitochondrial function is likely to be impaired.
Fatigue is a complex phenomenon, and it may be determined by several factors, including psychological health of the subjects. At the biochemical level fatigue is related to the metabolic energy available to an individual and ultimately to the many cells that perform their myriad of functions. The integrity of cell and intracellular membrane structures, especially in the mitochondria, is critical to cell function and energy production. If mitochondrial membrane glycophospholipids, fatty acids and other essential lipids are damaged by oxidation, they must be repaired or replaced in order to maintain cell and mitochondrial functions necessary in the production of cellular energy to combat fatigue.
The decline of energy production with aging appears to be due, in part, to mitochondrial lipid peroxidation by ROS and the failure to repair or replace the damaged molecules. Membrane damage and subsequent mitochondrial dysfunction by ROS can also lead to modifications (especially mutations and deletions) in mitochondrial DNA (mtDNA). The mitochondrial theory of aging proposes that the development of chronic degenerative diseases is the result, in part, of accumulated mtDNA mutations and deletions and oxidative damage to mitochondrial membranes over time.9,22,33 Indeed, these studies have linked the development of certain chronic diseases with the degree of mitochondrial membrane lipid peroxidation and mtDNA damage. Thus the damage to mtDNA and mitochondrial membranes seems to be involved in the etiology of age-associated degenerative diseases leading to changes in the expression of genes important for cell survival as well as the phenomenon of aging itself.33
Restoration of mitochondrial membrane integrity and fluidity are essential for the optimal functioning of the electron transport chain. Declines in energy production with aging and disease coupled with increases in oxidative stress can modify membrane lipids and increase mitochondrial membrane permeability and activate cellular death programs (apoptosis).34 Together these factors likely play a major role in the aging process and they also affect the development of age-related degenerative diseases.21,35
Some common antioxidants and accessory molecules used as dietary supplements
Some common antioxidants (incomplete list)
Vitamin C (ascorbic acid, buffered)
Vitamin E (alpha-tocopherol, other tocopherols, tocotrienols)
Coenzyme Q10
Alpha-Lipoic Acid (dihydrolipoate)
N-acetyl cysteine (also S-allyl cysteine, S-allyl cercaptocysteine)
Carotenoids/Oxycarotenoids (beta carotine, lycopene, lutein)
Flavanoids (quercetin, procyanidins, flavonols)
Proanthocyanidins
Selenium
Some other important accessory molecules (incomplete list)
Vitamin B3 (niacin)
Vitamin B6 (pyridoxine hydrochloride)
Vitamin B12 (cyanocobalamin)
Vitamin B2 (riboflavin)
Folic Acid (folate)
Melatonin
Magnesium
Zinc
Components of NT FactorTM
NT FactorTM is a nutrient complex that is extracted and prepared using a proprietary process. In addition, nutrients, vitamins and probiotic microorganisms are added to the preparation. It contains the following ingredients:
Glycophospholipids: polyunsaturated phosphatidylcholine, other polyunsaturated phosphatidyl lipids, glycolipids and essential fatty acids, including omega-3 and omega-6 fatty acids.
Probiotics: Bifido bacterium, Lactobacillus acidophilus and Lactobacillus bacillus in a freeze-dried, microencapsulated form with appropriate growth nutrients.
Food Supplements, Vitamins and Growth Media: Bacterial growth factors to support probiotic growth, including defatted rice bran, arginine, beet root fiber extract, black strap molasses, glycine, magnesium sulfate, para-amino-benzoate, leek extract, pantethine (bifidus growth factor), taurine, garlic extract, calcium borogluconate, artichoke extract , potassium citrate, calcium sulfate, spirulina, bromelain, natural vitamin E, calcium ascorbate, alpha-lipoic acid, oligosaccharides, vitamin B-6, niacinamide, riboflavin, inositol, niacin, calcium pantothenate, thiamin, vitamin B-12, folic acid, chromium picolinate.
OPTI Factor is Unique
OPTI Factor contains NT Factor® which is the most powerful proprietary substance to combat aging and fatigue.
How does NT Factor®, the nutrient compound found in OPTI Factor™, work?
“Aging, disease and degeneration place tremendous pressure on natural nutritional replacement processes. NT Factor® repairs damaged cells and restores their structure and function, thus it improves normal nutrient transport, communication and other functions to the states necessary for overall health and vitality”.
Dr. Garth Nicolson, Institute for Molecular Medicine
Every cell in your body contains dozens to thousands of minute structures (organelles) called mitochondria. These are the powerhouses of the cell, and are responsible for converting food and oxygen into the energy your body needs. Mitochondrial function or strength decreases with age, resulting in a loss of energy power, which leads to fatigue. Research has proven that the use of NT Factor repairs damaged cells, restores mitochondrial function and reduces mitochondrial function and reduces mitochondrial DNA damage, thereby reducing fatigue.
What can OPTI Factor™ do for you?
-
Dramatically reduces fatigue
-
Repairs damaged cells
-
Increases your energy
-
Cuts your biological age in half
All based on verified clinical research
What makes OPTI Factor™ different from any other product on the market today?
OPTI Factor protects and repairs the outer covering (membranes) of your cells from damage by a variety of causes. OPTI Factor rebuilds damaged membranes and restores electrical charges on those membranes that are important to transporting vital nutrients in and wastes out of your cells so that you can sustain life and enjoy youthful health and most importantly reduce fatigue.
OPTI Factor™ Repairs Damaged Cells
-
Replaces (damaged) phospholipids - the good fats that make up cell membranes
-
Improves the healthy function of cells, tissues and organs in your entire body
-
Normalizes membrane electrical activity needed for optimal performance
OPTI Factor™ … Proven clinically to cut your biological age in half.
Clinical studies have shown that the NT Factor®, the nutrient compound found in OPTI Factor™, dramatically decreases fatigue. Similar results were documented in another publication proving that fatigue was reduced in people with various illnesses. Besides increasing energy levels and defeating fatigue, OPTI Factor also increases mitochondrial function in aged people. In fact, within eight weeks of taking OPTI Factor, mitochondrial functionality of the aged population was enhanced to that of younger adults half their age.
This research validates the fact that OPTI Factor cuts your biological age in half. These findings are not only important to your cells but also to restoring and repairing all tissues, organs and systems in your entire body.
OPTI Factor Restores Mitochondrial Function
OPTI Factor contains NT Factor® which is the most powerful proprietary substance to combat aging and fatigue.
J Chronic Fatigue Syndr 2003; 11(3): 23-36
Nutritional Supplement (NT FactorTM) Restores Mitochondrial Function and Reduces Moderately Severe Fatigue in Aged Subjects
Michael Agadjanyan, PhD1, Vitaley Vasilevko, PhD1, Anahit Ghochikyan, PhD1,
Paul Berns, MD1, Patrick Kesslak, Ph.D.2,
Robert A. Settineri, MS3, and Garth L. Nicolson, PhD1
1. The Institute for Molecular Medicine, Huntington Beach, CA 92649
2. The Institute of Brain Aging and Dementia, University of California, Irvine, CA 92467
3. Research Consultant, Nutritional Therapeutics, Inc., Hauppauge, NY
ABSTRACT
OBJECTIVE: Decreased mitochondrial function is a characteristic of aging and fatigue. Here we determined if mild to moderately severe fatigue in a group of aged subjects (mean age >60 years), as defined by the validated Piper Fatigue Scale (PFS), can be significantly improved by use of a glycophospholipid dietary supplement, NT FactorTM (NTF). In addition, we determined if mitochondrial function, as defined by transport of the redox dye Rhodamine-123, is reduced in aging subjects with mild to moderately severe fatigue, and if this can be reversed with NTF supplementation in concert with improvement in fatigue scores.
METHODS: Participants who described a condition consistent with mild to moderately severe fatigue, as defined by the PFS, were examined by research nurse and completed a PFS survey form. The PFS rates fatigue from a score of 0 (no fatigue) to 10 (severe fatigue). Respondents who fulfilled the entry requirements were admitted to the study when their self-reported fatigue severity scores were rated as mild to moderately severe, and their fatigue could not be explained by an obvious clinical condition. Blood leukocytes were isolated for analysis of mitochondrial function by transport of Rhodomine-123, and the subjects were provided by open label the study product NTF. Twenty of the respondents (mean age= 68.9±4.18) completed the first part of the study on NTF for 12 weeks, and 16 of these subjects who agreed to discontinue the product also completed a wash-out period for an additional 12 weeks. Fatigue and mitochondrial function were determined every four weeks during the study.
RESULTS: There was a time-dependent reduction in overall fatigue in ten moderately fatigued subjects (average score 5.75±0.62, range 4.09-8.45) while on supplement but not in ten mildly fatigued subjects (average score 1.42±0.2, range 1.0-2.55). More specifically, after four weeks of NTF the average score of moderately fatigued subjects was reduced to 4.59 (20.2% reduction, p<0.005). Further use of NTF for a total of eight or twelve weeks decreased the overall average score of moderately fatigued subjects to 3.80±0.41 (33% reduction, p<0.001) or 3.71±0.48 (35.5% reduction, p<0.001), respectively, whereas in mildly fatigued subjects the fatigue scores were not significantly different. Analysis of mitochrondrial function indicated that four and eight weeks of NTF use in moderately fatigued subjects increased function by 15% and 26.8%, respectively, and restored mitochondrial function to levels similar to those found in young adults. Further use of NTF for a total of 12 weeks did not increase mitochondrial function as measured by the Rhodamine-123 assay. Some subjects were monitored 12 weeks after discontinuing use of NTF. Fatigue and mitochondrial function in moderately fatigued subjects were found to be intermediate between the initial findings and the results found at eight or 12 weeks of supplement use, indicating that continued use of NTF would be necessary to maintain lower fatigue scores and maintain mitochondrial function.
CONCLUSIONS: The dietary supplement with NTF reduced significantly moderate fatigue as measured by the Piper Fatigue Scale and significantly increased mitochondrial function in aged subjects.
INTRODUCTION
The most common complaint of patients in general medical practice is fatigue [1], and in fact, chronic fatigue is reported by 20% of all patients seeking medical care [1, 2]. Many well-known medical conditions are associated with chronic fatigue [3], and it is often an important secondary condition in many clinical diagnoses. Loss of energy and the symptom of fatigue often precede a clinical diagnosis, and this may be one reason that it is so commonly reported by patients seeking medical care.
Fatigue is thought to be a multidimensional sensation with many possible causes and no universally accepted definition. Piper et al. [4] described fatigue as a multi-component sensation with behavioral, affective, sensory and cognitive components. They also designed a simple measurement model that combined multiple fatigue-associated elements into an overall fatigue score [4].
At the cellular level fatigue is involved with cellular energy systems that for the most part are found in the mitochondria. Damage to cellular mitochondria can impair the abilities of cells to produce ATP and reduced NAD, and this occurs naturally with aging. Major targets of mitochondrial damage are phospholipid/protein membranes and mitochondrial DNA [5-7]. For example, damage of phospholipids in mitochondrial membranes by free radicals can affect membrane integrity, fluidity and transmembrane potentials, resulting in loss of energy production by the electron transport chain and its associated components. During the aging process mitochondria suffer damage to their membranes and DNA, and this is thought to contribute to or even be a cause of the aging process [8, 9].
Preventing cell membrane damage and loss of membrane integrity are important in prevention of loss of cellular energy. One method that has been used to replace damaged mitochondrial membrane phospholipids is replacement therapy, and this has been accomplished, in part, by replacement of damaged lipids using a dietary supplement containing polyunsaturated phosphatidylcholines and other phospholipids and fatty acids that are essential structural and functional components of all biological membranes [10-12].
In previous studies a dietary supplement, NT FactorTM (NTF) in a vitamin and mineral mixture (PropaxTM), was used to reduce chemotherapy-induced fatigue, nausea, vomiting and other side effects associated with chemotherapy [10]. NTF was also used to protect from hearing loss associated with aging and prevent mitochondrial membrane potential changes and mitochondrial DNA deletions that occur with aging [11]. We used Propax plus NTF in a pilot study with severely fatigued, aged subjects to reduce fatigue, as measured by the Piper Fatigue Scale (PFS). We found that fatigue was reduced 33%, from severe to moderate fatigue, after eight weeks of using PropaxTM containing NTF [12]. The present study was initiated to examine the effects of NTF on fatigue in moderately and mildly fatigued subjects and to determine if their mitochondrial function, as measured by the transport and reduction of Rhodamine-123 [13], improved with administration of NTF in concert with improvements in fatigue scores.
SUBJECTS AND METHODS
Subjects: Participants were prescreened on the basis of an initial phone conversation to determine whether their symptoms were consistent with persistent, intractable fatigue, or merely an intermittent condition linked to their work or lifestyle. Those who described a condition consistent with the definition of fatigue as defined in the Piper Fatigue Scale (PFS) [4] were mailed a survey form. This instrument defines fatigue as an unusual sense of tiredness that is not usually relieved by either a good night’s sleep or by rest. The completed, returned surveys were then scored as described previously [12].
After the initial PFS survey, participants aged 60 years and older with an overall fatigue score of 1 to 7 were examined by a research nurse and admitted to this pilot study if their fatigue could not be explained by a pre-existing clinical condition. The participants were divided into two groups: score 1-4 or mild fatigue and score 4-7 or moderate fatigue on a scale of 1-10 (0 = no fatigue, 1-4 = mild fatigue, 4-7 = moderate fatigue, 7-10 = severe fatigue).
There were 20 respondents who fully completed the study that had an average age of 68.9±4.8, with a range of 61-77. There were seven men whose average age was 67.6±3.15, with a range of 61-71 and thirteen women whose average age was 69.5±4.61, with a range of 62-77. All of the subjects were from Southern California. Subjects were asked if they used any prescription medications. Nine participants or 45% used prescription medications (Table 1). However, of the nine subjects in Table 1 who indicated persistent, intractable fatigue, only four used more than one medication. All who listed depression as a diagnosis were on antidepressants, and of the four hypothyroid respondents three were on Armour Thyroid supplementation.
Study Design: Subjects signed an informed consent document and were admitted into the study with mild (1-4 on the PFS) or moderate fatigue (4-7 on the PFS). Each participant was given instructions to use three tablets of NT factorTM twice daily. The identity of the product that the participants were to take during the trial was not identified on the label, and it was given to subjects in plain bottles with instructions clearly marked on the label. Their blood was taken for analysis, and they were provided a four-week supply of NTF and told to return after the fourth week of using the product. If a blood chemistry panel (Chem-20) indicated that the subject had values outside the normal range, they were excluded from the study. All subjects repeated the PFS assessment at the end of the fourth, eighth and 12th week when they returned for collection of blood. After the 12th week, the participants stopped using NTF. These subjects returned after the 24th week (12 week wash-out period). At that time blood was drawn, the participants completed their PFS questionnaires, and all of the forms were checked for verification, completion and scoring accuracy [12].
Materials and Methods: The supplement product, NT FactorTM (Nutritional Therapeutics, Inc., Hauppauge, NY), is a proprietary vitamin, mineral and nutrient complex containing an exogenous source of polyunsaturated phosphatidylcholine and other membrane phospholipids (Table 2) [12]. The participants took the product twice daily for 12 weeks. Each four weeks the participants returned the product container for determination of compliance. After 12 weeks the participants discontinued use of the product, and 12 weeks later they were retested.
The PFS is composed of 22 numerically scaled questions rated from 0 (no fatigue) to 10 (severe) fatigue. These items measure four dimensions of subjective fatigue: behavioral/severity (6 items); affective/meaning (5 items); sensory (5 items); and cognitive/mood (6 items). These are used to calculate the four sub-scale/dimensional scores and the total fatigue scores. The standardized alpha (Cronbach’s alpha) did not drop below 0.90 for any of the subscales, and the standard alpha for the entire scale of 22 questions was 0.96, indicating excellent reliability for an established instrument [14].
Mitochondrial function was determined by transport and reduction of the dye Rhodamine-123 as described previously [13]. Peripheral blood mononuclear cells (PBMC) were isolated from whole blood using a Ficoll-Hypaque gradient by centrifugation at 1,800 rpm for 30 min in a clinical centrifuge at room temperature. PBMC were stained with 2.0 or 10 mM of Rhodamine-123 (Sigma Chemical, St. Louis, MO) in phosphate-buffered saline (PBS) in the dark for 15 min at 370C. To remove unbound dye prior to flow cytometric analysis the cells were washed twice by centrifugation in ice-cold PBS and re-suspended in cold PBS. Non-viable cells were excluded from analysis using a light scatter gate established by staining with a propidium iodide (Sigma Chemical) solution. Data was collected on Rhodamine-123 fluorescence using an argon ion laser tuned at 488 nm (FACScan, Becton Dickinson, Mountain View, CA) and analyzed using CellQuest software (Becton Dickinson). Since the data using 2.0 or 10 mM of Rhodamine-123 were similar, only the data using the 10 mM dose was reported. Results from the mitochondrial staining with Rhodamine-123 were analyzed using a repeated measures analysis of variance (ANOVA) and Bonferroni/Dunn post-hoc test for specific group differences (young control, mild versus moderate fatigue, treatment times, washout, etc.).
RESULTS
NTF improved the overall fatigue scores of moderately fatigued subjects as measured by the PFS (Table 3). The initial PFS group average (mean) fatigue score was 5.75±0.6, and after four weeks of NTF this improved to 4.59±0.5 or a 20.2% reduction in fatigue. After eight and 12 weeks of NTF the PFS fatigue scores of the moderately fatigued group improved to 3.8±0.6 (33% reduction) and 3.71±0.65 (35.5% reduction), respectively (Table 3). These changes were significant (p<0.001). By sex, the total PFS mean score improved in moderately fatigued subjects after taking NTF for four weeks by 15.3% in women and 23.5% in men. After eight and 12 weeks, fatigue improved in women by 27.5% and 32.3%, respectively, and in men by 40.5% and 42.9%, respectively. There were no significant differences between the results with men and women. In contrast to moderately fatigued subjects, however, NTF use did not have a significant effect on mild fatigue. The improvement in fatigue scores overall after 12 weeks of NTF in mildly fatigued subjects was only 5.6% (Table 3). As found previously with severely fatigued subjects [12], age was not associated with the degree of change in fatigue in the moderately fatigued group using the NTF supplement. When subjects stopped using NTF, their fatigue scores increased. Twelve weeks after stopping NTF the moderately fatigued group had fatigue scores of 4.53±0.4 or 21.2% difference with the baseline value, whereas in the mildly fatigued group there were no significant differences in overall PFS fatigue scores (Table 3).
Using the PFS subscales the Behavioral/Severity scores improved in moderately fatigued subjects after 12 weeks of NTF an average of 15.3% (Table 3). The Affective/Meaning subscale improved by an average of 37% and 42.7% after NTF use for 8 and 12 weeks, respectively. The Sensory subscale revealed 37.9% and 40.5% average improvements for the group after 8 and 12 weeks NTF use, respectively. Finally, the Cognitive/Mood subscale showed average improvements of 37.2% and 32.9% after 8 and 12 weeks of NTF use, respectively (Table 3).
Mitochondrial function was measured in the various groups using the Rhodamine-123 assay. Since the data using 2 or 10 mM Rhodamine-123 were not significantly different, only the data using 10 mM dye was reported. The staining of mitochondria with Rhodamine-123 changed significantly throughout the course of treatment of both moderately and mildly fatigued subjects with NTF (Figures 1 and 2) (F [4,76]=29.917, p<0.0001). Post-hoc analysis with the Bonferroni/Dunn test for specific differences between groups indicated that after 8 and 12 weeks of NTF the results were significantly different from baseline (p<0.0001) and after washout for an additional 12 weeks (p<0.001); however, there was no significant difference between the use of NTF for 8 and 12 weeks (p>0.4). After 12 weeks of NTF the Rhodamine-123 mitochondrial assay yielded results similar to and not significantly different from those found in non-fatigued young adults that had not taken NTF (Figures 1 and 2). In the moderately fatigued group 8 or 12 weeks of NTF use resulted in significant differences (p<0.001) in mitochondrial function; however, there was no significant difference between the 8- and 12-week groups (p>0.17) (Figure 1). This amounted to an increase in mitochondrial function by 8.4%, 23.8% and 23.7%, respectively, after four, eight and 12 weeks of NTF use in moderately fatigued subjects (Figure 1). Some subjects were monitored 12 weeks after discontinuing use of NTF. Although still significantly different from baseline (p<0.001), mitochondrial function returned to intermediate values between baseline and the values at 8 or 12 weeks (Figures 1 and 2). When analyzed by sex, there were no significant differences between men and women in any of the groups.
DISCUSSION
Mitochondria are the most important source of cellular energy in our bodies. If their function is impaired, energy available to cells is limited to the Krebs Cycle. There are a number of conditions and substances that can impair mitochondrial function [5-8], but oxidation and damage of mitochodrial lipids in membranes are among the most important causes of impairment of mitochondrial function. This may result in modification of the electrical potential barrier across the mitochondrial membranes that is essential in the electron transport chain generation of cellular energy molecules. The dietary supplement NTF used in this pilot study is a unique mixture of cellular lipids that is rich in phospholipids and glycophospholipids, and in particular, polyunsaturated phosphatidylcholine and other membrane lipids. It also contains essential fatty acids and other lipids that are important in mitochondrial function and cellular membrane health and probiotic microorganisms to aid in intestinal uptake [12].
NTF has been used in clinical trials on cancer patients, and it has been shown to cause a substantial positive impact on fatigue. In a twelve week double-blinded, cross-over, placebo controlled, randomized trial on cancer patients receiving chemotherapy NTF in a vitamin-mineral supplement (PropaxTM) showed improvement from fatigue, nausea, diarrhea, impaired taste, constipation, insomnia and other quality of life indicators [10]. Most (64%) of the patients in the study reported significant improvement in these and other chemotherapy-induced side effects, and 29% experienced no overall worsening of side-effects. Following cross-over to the supplement containing NTF patients reported rapid improvement in nausea, impaired taste, tiredness, appetite, sick feeling and other indicators.
NTF has also demonstrated an anti-aging effect on hearing loss in aging rats. Using 18-20 month-old Harlan-Fisher 344 rats Seidman et al. [11] found that NTF prevented hearing loss associated with aging, shifting the threshold hearing from 35-40 dB in control aged animals to 13-17 dB in the test group. These results were found to be significant (p<0.005). They also found that NTF preserved cochlear mitochondrial function as measured in the Rhodamine-123 transport assay, increasing mitochondrial function by 34%. NTF also prevented the common aging-related mitochondrial DNA deletion (mtDNA4834) found in the cochlear of aging rats.
We also found an effect of PropaxTM with NTF in a pilot trial designed to measure fatigue in aged patients (>50 years-old) with a variety of common clinical conditions [12]. In these severely fatigued subjects (mean PFS scores = 7.9±0.82) NTF significantly reduced fatigue to moderate levels. After eight weeks of NTF there was a 40% reduction in overall fatigue (mean PFS scores = 4.7±2.01) as measured by the FPS instrument. These results are comparable to the data presented here for moderately fatigued subjects, where we found a 35.5% reduction in overall fatigue in moderately fatigued subjects after eight weeks use of NTF.
In the current study we utilized moderately (PFS scale=4-7) and mildly fatigued (PFS scale=1-4) subjects but only found a significant effect on fatigue in the moderately fatigued group of either sex. There could be a number of reasons for this observation, but it is unlikely that the only contribution to fatigue in these patients is mitochondrial function. Fatigue is a complex phenomenon, and it may be determined by several factors, including psychological health of the subjects. Also, in the mildly fatigued patients differences are difficult to determine because of the nature of the measuring instrument, and it might be unreasonable to expect significant differences in subjects that score very low initially on the PFS.
Our subjects were not randomly chosen for this study; they were recruited using a health talk radio program in the Los Angeles, CA region. The only criteria was that they were older than 50 years-old, mildly to moderately fatigued (using the PFS scales), and their fatigue could not be explained by an underlying clinical condition. Subjects were given a physical examination by a research nurse, and their blood was analyzed using a standard chemistry profile for possible clinical problems. This procedure did eliminate some prospective subjects from the trial. The subjects that qualified for the trial used NTF supplement for 12 weeks, and most of them then went off product for an additional 12 weeks to see if they would return to baseline fatigue and mitochondrial function values. Another potential problem was the number of participants with mild or moderate fatigue used in the study. Ideally, we would have liked to have a larger number of participants, but a number of factors prevented this, including the cost of the study.
Fatigue is related to the metabolic energy available to an individual and ultimately to the many cells that perform their myriad of functions. The integrity of cell and intracellular membrane structures, especially in the mitochondria, is critical to cell function and energy production [15-17]. NTF provides cells and mitochondria with the glycophospholipids, fatty acids and other essential lipids to repair and replace membrane components needed for maintenance of cell and mitochondrial function necessary in the production of cellular energy to combat fatigue.
The decline of energy production with aging may be due, in part, to mitochondrial lipid peroxidation by reactive oxygen species. Membrane damage and subsequent mitochondrial dysfunction can also lead to modifications (especially mutations and deletions) in mitochondrial DNA (mtDNA). The mitochondrial theory of aging proposes that the development of chronic degenerative diseases is the result, in part, of accumulated mtDNA mutations and deletions and oxidative damage to mitochondrial membranes over time. Indeed, some studies have linked the development of certain chronic diseases with the degree of mitochondrial membrane lipid peroxidation and mtDNA damage. Thus the damage to mtDNA and mitochondrial membranes seems to be involved in the etiology of age-associated degenerative diseases leading to changes in the expression of genes important for cell survival as well as the phenomenon of aging itself [18]. Restoration of mitochondrial membrane integrity and fluidity are essential for the optimal functioning of the electron transport chain [19]. Declines in energy production with aging coupled with an increase in oxidative stress can modify membrane lipids and increase mitochondrial membrane permeability and activate cellular death programs (apoptosis). Together these factors likely play a major role in the aging process and they also affect the development of age-related degenerative diseases [20].
The first outward sign of cellular deterioration may be fatigue. As the phospholipid structure of the mitochondrial membrane loses fluidity and becomes more porous at lipid/protein interfaces in the membrane, the membrane potential is affected and less able to maintain the electron transport process. In addition, the electron transport chain increases the production of Reactive Oxygen Species (ROS), free radicals that can further damage mitochondrial membranes and mtDNA. Although there is always some inherent mitochondrial membrane leakage and damage, this is usually repaired, unless the rate of repair is exceeded by the rate of oxidative damage [21].
Finally, since ROS are highly implicated in age-associated mtDNA damage, we tried to determine the age-dependent accumulation of a particular 4977 bp mtDNA deletion in platelets of patients. The 83 bp fragment of deleted mtDNA was detected in all samples including control blood from young volunteers. The amount of the deletion in blood cells did not show an age-dependent increase, and differences in amounts (quantity was estimated by fluorescence intensity of ethidium bromide stained DNA in agarose gel) of deletion were detected in blood before and after NTF use (data not shown). The 4977 bp deletion in mtDNA is known to accumulate with age in various human postmitotic tissues, such as brain, heart and skeletal muscle. Different groups have tried to use blood cells as a possible model for screening the accumulation of mtDNA mutations; however, the results have so far been contradictory. Biagini et al. [22] as well as the other groups [23-26] failed to detect this particular deletion in blood or platelets both from young and old individuals, whereas Meissner et al. [23] demonstrated that this deletion is detectable in blood cells, but the amount is substantially lower than in postmitotic tissues. Also Meissner et al. [23] did not show an age-dependent increase in the 4977 bp deletion in mtDNA. Our data are in good accordance with the assertion of Meissner et al. [23] that the accumulation of 4977 bp mtDNA deletion in blood cells is not age-dependent, and this might be explained by higher turnover rate of blood cells.
Acknowledgments
We acknowledge the excellent assistance of Christy Bennet and Ned Realiza. This study was supported by a grant from Nutritional Therapeutics, Inc. Dr. Berns was previously a consultant for Nutritional Therapeutics, Inc.
References
1. Kroenke K, Wood DR, Mangelsdorff AD, Meier NJ, Powell JB. Chronic fatigue in primary care. Prevalence, patient characteristics, and outcome. JAMA. 1988; 260: 929-934.
2. Morrison JD. Fatigue as a presenting complaint in family practice. J Family Pract 1980; 10: 795-801.
3. McDonald E, David AS, Pelosi AJ, Mann AH. Chronic fatigue in primary care attendees. Psychol Med. 1993; 23: 987-998
4. Piper BF, Linsey AM, Dodd MJ. Fatigue mechanism in cancer. Oncol Nursing Forum. 1987; 14: 17-23.
5. Richter C, Par JW, Ames B. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc Nat Acad Sci. USA 1998; 85: 6465-6467.
6. Wei YH, Lee HC. Oxidative stress, mitochondrial DNA mutation and impairment of antioxidant enzymes in aging. Exp Biol Med. 2002; 227:671-682.
7. Spector AA, Yorek MA. 1985. Membrane Lipid composition and cellular function. J Lipid Res. 1985; 26:10105.
8. Harman D. Aging: A theory based on free radical and radiation chemistry. J Gerontol. 1956; 2: 298-300.
9. Xu D, Finkel T. A role for mitochondria as potential regulators of cellular life span. Biochem Biophysics Res Commun 2002; 294:245-248.
10. Colodny L, Lynch K, Farber C, Papish S, et al. Results of a study to evaluate the use of Propax to reduce adverse effects of chemotherapy. JANA 2000; 2: 17-25.
11. Seidman M, Khan MJ, Tang WX, Quirk WS. Influence of lecithin on mitochondrial DNA and age-related hearing loss. Otolaryngol Head Neck Surg 2002; 127:138-144.
12. Ellithorpe RR, Settineri R, Nicolson GL. Pilot Study: Reduction of fatigue by use of a dietary supplement containing glycophospholipids. JANA 2003; in press.
13. Kim MJ, Cooper DD, Hayes SF, Spangrude GJ. Rhodamine-123 staining in hematopoietic stem cells of young mice indicates mitochondrial activation rather than dye efflux. Blood 1998; 91: 4106-4117.
14. Nunnally JC. 1978. Psychometric Theory (2nd ed.) New York: McGraw-Hill, pp. 117-123.
15. Zeisel SH, In Hanin I, Popell G, (eds.) 1996. Phospholipids, biochemical pharmaceutical and analytical considerations. New York: Plenum Press, pp. 219-231.
16. Conlay LA, Wurtman RJ, Blusztajn K, Coviella IL, Maher TJ, Evoniak GE. N Engl J Med. 1986; 175: 892.
17. Johns DR. 1995. Seminars in medicine of Beth Israel Hospital, Boston: Mitochondrial DNA and Disease. N Engl J Med. 1995; 333: 638-44.
18. Kowald A. The mitochondrial theory of aging: do damaged mitochondria accumulate by delayed degradation? Exp Gerontol 1999; 34:605-612.
19. Paradies G, Petrosillo G, Pistolese M, Ruggiero F. Reactive oxygen species affect mitochondrial electron transport complex I activity through oxidative cardiolipin damage. Gene 2002; 286:135-141.
20. Lin M, Simon D, Ahn C, Lauren K, Beal MF. High aggregrate burden of somatic mtDNA point mutations in aging and Alzheimer’s disease brain. Human Mol Genet 2002; 11:133-145.
21. Koboska J, Coskun P, Esposito L, Wallace DC. Increased mitochondrial oxidative stress in the Sod2(+/-) mouse results in age-related decline of mitochondrial function culminating in increased apoptosis. Proc Nat Acad Sci USA 2001; 98:2278-2283.
22. Biagini G, Pallotti F, Carraro S, Sgarbi G, Pich MM, Lenaz G, Anzivino F, Gualandi G, Xin D.
Mitochondrial DNA in platelets from aged subjects. Mech Ageing Dev 1998; 101:269-275.
23. Meissner C, Mohamed SA, Klueter H, Hamann K, von Wurmb N, Oehmichen M. Quantification of mitochondrial DNA in human blood cells using an automated detection system. Forensic Sci Int 2000; 113:109-112.
24. Lee HC, Oang CY, Hsu HS, Wei YH. Deletion in blood mitochondrial DNA in Kearns-Sayre syndrome. Biochim Biophys Acta 1994; 1226:37-43.
25. Poulton J, Deadman ME, Ramacharan S, Gardiner RM. Germ-line deletions of mtDNA in mitochondrial myopathy. Am J Hum Genet 1991; 90:649-653.
26. Smith OP, Hamm MJ, Woodward CE, Brockington M. Pearson’s marrow/pancreas syndrome: haematological features associated with deletion and duplication of mitochondrial DNA. Br J Haemotol 1995; 90:469-472.
Table 2. Components of NT FactorTM
NT FactorTM is a nutrient complex that is extracted and prepared using a proprietary process. In addition, nutrients, vitamins and probiotic microorganisms are added to the preparation. It contains the following ingredients
Glycophospholipids: polyunsaturated phosphatidylcholine, other polyunsaturated phosphatidyl lipids, glycolipids and essential fatty acids, including omega-3 and omega-6 fatty acids.>
Probiotics: Bifido bacterium, Lactobacillus acidophilus and Lactobacillus bacillus in a freeze-dried, microencapsulated form with appropriate growth nutrients.>
Food Supplements, Vitamins and Growth Media: Bacterial growth factors to support probiotic growth, including defatted rice bran, arginine, beet root fiber extract, black strap molasses, glycine, magnesium sulfate, para-amino-benzoate, leek extract, pantethine (bifidus growth factor), taurine, garlic extract, calcium borogluconate, artichoke extract , potassium citrate, calcium sulfate, spirulina, bromelain, natural vitamin E, calcium ascorbate, alpha-lipoic acid, oligosaccharides, vitamin B-6, niacinamide, riboflavin, inositol, niacin, calcium pantothenate, thiamin, vitamin B-12, folic acid, chromium picolinate.
FATIGUE
OPTI Factor and Fatigue Reduction
OPTI Factor with the NT Factor® Nutrient Compound
Clinically Proven to Reduce Fatigue by 40% in Eight Weeks
OPTI Factor is a proprietary blend of phospholipids and glycolipids whose mechanism of action is to repair cellular membranes by increasing cell membrane fluidity. By repairing the membranes of the mitochondria (the energy furnaces within our cells), we allow all of our cells to increase their nutrient uptake so that the mitochondria may product more ATP --- the body's energy fuel.
40% Fatigue Reduction: This study was conducted on people with moderate and severe fatigue. Within eight weeks, there was a 40% reduction in fatigue, as measured by the Piper Fatigue Scale. The research measured results at 4 weeks, 8 weeks and 12 weeks. The research indicated a loading dose over the first two months, followed by a maintenance dose thereafter.
Research Paper 40% Reduction in Fatigue Click Here
(This research relates to NT Factor® the proprietary ingredient in OPTI Factor)
Reversal of Age-Related Energy Loss: This study compared the mitochondrial function of two groups, with one being of average age 69 and the other average age 29. Within eight weeks, the mitochondrial function of the older group was increased to that of the younger group. Unlike antioxidants which may prevent additional damage, NT Factor actually repaired the cell membranes and resulted in increased energy.
Research Paper * Reversal of Age-Related Energy Loss Click Here
(This research relates to NT Factor® the proprietary ingredient in OPTI Factor)
Mitochondria are the primary energy producers in the body. Mitochondria are the energy furnaces of each cell, with the typical cell possessing an average of 1,000 mitochondria. Given that there are 10 to the 13th power cells in a healthy adult, healthy and productive mitochondria are critical to maintain the body's energy and resilience. Recent studies have shown the importance to maintaining healthy MTDNA, the DNA which resides inside the mitochondria. Unlike standard DNA which has histones and introns to protect it from free radical damage, the mitochondrial DNA does not have this protective mechanism so it is much more exposed and more susceptible to oxidative damage (ROS or reactive oxidative stress). And since mitochondrial cell division takes place approximately every five to six days and replicates the damage existing in the old cells, the damaged MTDNA spreads rapidly. This is what eventually depletes the patient's energy level and may lead to abnormal cellular formation.
Fatigue Facts
Fatigue is a common health complaint. It is one of the hardest terms to define, and a symptom of many different conditions.
Fatigue, also known as weariness, tiredness, exhaustion, or lethargy, is generally defined as a feeling of lack of energy.
Fatigue is not the same as drowsiness, but the desire to sleep may accompany fatigue. Apathy is a feeling of indifference that may accompany fatigue or exist independently.
Fatigue is common. Around 20% of Americans claim to have fatigue intense enough to interfere with their having a normal life. Physical causes are estimated at 20-60%, and emotional causes are the other 40-80%. The challenge is how to tell what is causing your fatigue and whether it is serious enough to see your doctor.
Fatigue Causes
Lifestyle. Feelings of fatigue often have an obvious cause, such as sleep deprivation, overwork or unhealthy habits. Psychological. Fatigue is a common symptom of mental health problems, such as depression and grief, and may be accompanied by other signs and symptoms, including irritability and lack of motivation. Medical. Unrelenting exhaustion may be a sign of an underlying illness, such as a thyroid disorder, heart disease or diabetes.
Common Causes of Fatigue Include:
- Acute liver failure
- Alcohol use or abuse
- Anemia
- Anxiety
- Caffeine use
- Cancer
- Chronic fatigue syndrome
- COPD
- Depression (major depression)
- Emphysema
- Excessive physical activity
- Grief Heart disease
- Hyperthyroidism (overactive thyroid)
- Hypothyroidism (underactive thyroid)
- Inactivity Kidney failure, chronic
- Lack of sleep
- Medications, such as antihistamines, cough and cold remedies, prescription pain medications, heart medications, blood pressure medications, and some antidepressants
- Obesity
- Pregnancy
- Recovery from major surgery
- Restless legs syndrome
- Sleep apnea
- Stress
- Type 1 diabetes
- Type 2 diabetes
- Unhealthy eating habits
Fatigue Symptoms
Symptoms of Fatigue Include:
- Weakness, lack of energy, tiredness, exhaustion
- Passing out or feeling as if you are going to pass out
- Palpitations (feeling your heart beating)
- Dizziness
- Vertigo
- Shortness of breath
When to Seek Medical Care
Generally, you need to see your doctor if you experience any of the following:
- Fatigue that comes on suddenly
- Fatigue that is not relieved by adequate rest, adequate sleep, or removal of stressful factors
- Fatigue that is accompanied by unexplained symptoms
- Feeling as if you are going to pass out
- Unexplained weight changes
- Menstrual irregularities
- Any new masses, lumps, or bumps
- Mild to moderate pain in your head, chest, or abdomen
If you experience any of the following, you should go to a hospital’s Emergency Department:
- Pass out
- Chest pain
- Shortness of breath
- Bleeding - Rectal bleeding, vomiting blood
- Severe abdominal, pelvic, or back pain
- Severe headache
- Irregular or fast heartbeat
- Other people or pets in same household have same symptoms (possible carbon monoxide poisoning)
Fatigue Treatment
Many causes of fatigue may be treated with medications.
- Iron supplements for anemia
- Medications and machines to help sleep apnea
- Medications to control your blood sugar
- Medications to support your thyroid
- Antibiotics to treat infection
- Vitamins
- Recommendations for dietary changes and a sensible exercise program
Prevention
- Manage your stress: Practice relaxation. Take time out for yourself.
- Get exercise: Start slowly. Do something you like. Find a good time to exercise. Find a partner.
- Check out your medications.
- Improve your diet: Eat a good breakfast (whole grain cereal, fruit, milk). Add more fruits and vegetables.
- Stop the caffeine habit.
- Give up smoking.
- Have sex with your spouse or partner.
- Get enough sleep: Have a routine. Go to bed at the same time every night.
- Avoid coffee, tea, or caffeinated drinks after 6 pm.
- Drink no alcohol after dinner and decrease the total amount of alcohol you consume.
OPTI Factor and Fatigue in Aging
OPTI Factor contains NT Factor® daily which is the most powerful proprietary substance to combat aging and fatigue.
Authors: Michael Agadjanyan, PhD, Vitaley Vasilevko, PhD, Anahit Ghochikyan, PhD, Paul Berns, MD, Patrick Kesslak, PhD, Robert A. Settineri, MS and Garth L. Nicolson, PhD
OBJECTIVE: Decreased mitochondrial function is a characteristic of aging and fatigue. Here we determined if mild to moderately severe fatigue in a group of aged subjects (mean age >60 years), as defined by the validated Piper Fatigue Scale (PFS), can be significantly improved by use of a glycophospholipid dietary supplement, NT Factor™ (NTF). In addition, we determined if mitochondrial function, as defined by transport of the redox dye Rhodamine-123, is reduced in aging subjects with mild to moderately severe fatigue, and if this can be reversed with NTF supplementation in concert with improvement in fatigue scores.
METHODS: Participants who described a condition consistent with mild to moderately severe fatigue, as defined by the PFS, were examined by research nurse and completed a PFS survey form. The PFS rates fatigue from a score of 0 (no fatigue) to 10 (severe fatigue). Respondents who fulfilled the entry requirements were admitted to the study when their self-reported fatigue severity scores were rated as mild to moderately severe, and their fatigue could not be explained by an obvious clinical condition. Blood leukocytes were isolated for analysis of mitochondrial function by transport of Rhodomine-123, and the subjects were provided by open label the study product NTF. Twenty of the respondents (mean age= 68.9±4.18) completed the first part of the study on NTF for 12 weeks, and 16 of these subjects who agreed to discontinue the product also completed a wash-out period for an additional 12 weeks. Fatigue and mitochondrial function were determined every four weeks during the study.
RESULTS: There was a time-dependent reduction in overall fatigue in ten moderately fatigued subjects (average score 5.75±0.62, range 4.09-8.45) while on supplement but not in ten mildly fatigued subjects (average score 1.42±0.2, range 1.0-2.55). More specifically, after four weeks of NTF the average score of moderately fatigued subjects was reduced to 4.59 (20.2% reduction, p<0.005). Further use of NTF for a total of eight or twelve weeks decreased the overall average score of moderately fatigued subjects to 3.80±0.41 (33% reduction, p<0.001) or 3.71±0.48 (35.5% reduction, p<0.001), respectively, whereas in mildly fatigued subjects the fatigue scores were not significantly different. Analysis of mitochrondrial function indicated that four and eight weeks of NTF use in moderately fatigued subjects increased function by 15% and 26.8%, respectively, and restored mitochondrial function to levels similar to those found in young adults. Further use of NTF for a total of 12 weeks did not increase mitochondrial function as measured by the Rhodamine-123 assay. Some subjects were monitored 12 weeks after discontinuing use of NTF. Fatigue and mitochondrial function in moderately fatigued subjects were found to be intermediate between the initial findings and the results found at eight or 12 weeks of supplement use, indicating that continued use of NTF would be necessary to maintain lower fatigue scores and maintain mitochondrial function.
CONCLUSIONS: The dietary supplement with NTF reduced significantly moderate fatigue as measured by the Piper Fatigue Scale and significantly increased mitochondrial function in aged subjects.
………………………………………………………………………………………
INTRODUCTION
The most common complaint of patients in general medical practice is fatigue [1], and in fact, chronic fatigue is reported by 20% of all patients seeking medical care [1, 2]. Many well-known medical conditions are associated with chronic fatigue [3], and it is often an important secondary condition in many clinical diagnoses. Loss of energy and the symptom of fatigue often precede a clinical diagnosis, and this may be one reason that it is so commonly reported by patients seeking medical care.
Fatigue is thought to be a multidimensional sensation with many possible causes and no universally accepted definition. Piper et al. [4] described fatigue as a multi-component sensation with behavioral, affective, sensory and cognitive components. They also designed a simple measurement model that combined multiple fatigue-associated elements into an overall fatigue score [4].
At the cellular level fatigue is involved with cellular energy systems that for the most part are found in the mitochondria. Damage to cellular mitochondria can impair the abilities of cells to produce ATP and reduced NAD, and this occurs naturally with aging. Major targets of mitochondrial damage are phospholipid/protein membranes and mitochondrial DNA [5-7]. For example, damage of phospholipids in mitochondrial membranes by free radicals can affect membrane integrity, fluidity and transmembrane potentials, resulting in loss of energy production by the electron transport chain and its associated components. During the aging process mitochondria suffer damage to their membranes and DNA, and this is thought to contribute to or even be a cause of the aging process [8, 9].
Preventing cell membrane damage and loss of membrane integrity are important in prevention of loss of cellular energy. One method that has been used to replace damaged mitochondrial membrane phospholipids is replacement therapy, and this has been accomplished, in part, by replacement of damaged lipids using a dietary supplement containing polyunsaturated phosphatidylcholines and other phospholipids and fatty acids that are essential structural and functional components of all biological membranes [10-12].
In previous studies a dietary supplement, NT Factor™ (NTF) in a vitamin and mineral mixture (Propax™), was used to reduce chemotherapy-induced fatigue, nausea, vomiting and other side effects associated with chemotherapy [10]. NTF was also used to protect from hearing loss associated with aging and prevent mitochondrial membrane potential changes and mitochondrial DNA deletions that occur with aging [11]. We used Propax plus NTF in a pilot study with severely fatigued, aged subjects to reduce fatigue, as measured by the Piper Fatigue Scale (PFS). We found that fatigue was reduced 33%, from severe to moderate fatigue, after eight weeks of using Propax™ containing NTF [12]. The present study was initiated to examine the effects of NTF on fatigue in moderately and mildly fatigued subjects and to determine if their mitochondrial function, as measured by the transport and reduction of Rhodamine-123 [13], improved with administration of NTF in concert with improvements in fatigue scores.
SUBJECTS AND METHODS
Subjects: Participants were prescreened on the basis of an initial phone conversation to determine whether their symptoms were consistent with persistent, intractable fatigue, or merely an intermittent condition linked to their work or lifestyle. Those who described a condition consistent with the definition of fatigue as defined in the Piper Fatigue Scale (PFS) [4] were mailed a survey form. This instrument defines fatigue as an unusual sense of tiredness that is not usually relieved by either a good night’s sleep or by rest. The completed, returned surveys were then scored as described previously [12].
After the initial PFS survey, participants aged 60 years and older with an overall fatigue score of 1 to 7 were examined by a research nurse and admitted to this pilot study if their fatigue could not be explained by a pre-existing clinical condition. The participants were divided into two groups: score 1-4 or mild fatigue and score 4-7 or moderate fatigue on a scale of 1-10 (0 = no fatigue, 1-4 = mild fatigue, 4-7 = moderate fatigue, 7-10 = severe fatigue).
There were 20 respondents who fully completed the study that had an average age of 68.9±4.8, with a range of 61-77. There were seven men whose average age was 67.6±3.15, with a range of 61-71 and thirteen women whose average age was 69.5±4.61, with a range of 62-77. All of the subjects were from Southern California. Subjects were asked if they used any prescription medications. Nine participants or 45% used prescription medications (Table 1). However, of the nine subjects in Table 1 who indicated persistent, intractable fatigue, only four used more than one medication. All who listed depression as a diagnosis were on antidepressants, and of the four hypothyroid respondents three were on Armour Thyroid supplementation.
Study Design: Subjects signed an informed consent document and were admitted into the study with mild (1-4 on the PFS) or moderate fatigue (4-7 on the PFS). Each participant was given instructions to use three tablets of NT factor™ twice daily. The identity of the product that the participants were to take during the trial was not identified on the label, and it was given to subjects in plain bottles with instructions clearly marked on the label. Their blood was taken for analysis, and they were provided a four-week supply of NTF and told to return after the fourth week of using the product. If a blood chemistry panel (Chem-20) indicated that the subject had values outside the normal range, they were excluded from the study. All subjects repeated the PFS assessment at the end of the fourth, eighth and 12th week when they returned for collection of blood. After the 12th week, the participants stopped using NTF. These subjects returned after the 24th week (12 week wash-out period). At that time blood was drawn, the participants completed their PFS questionnaires, and all of the forms were checked for verification, completion and scoring accuracy [12].
Materials and Methods: The supplement product, NT Factor™ (Nutritional Therapeutics, Inc., Hauppauge, NY), is a proprietary vitamin, mineral and nutrient complex containing an exogenous source of polyunsaturated phosphatidylcholine and other membrane phospholipids (Table 2) [12]. The participants took the product twice daily for 12 weeks. Each four weeks the participants returned the product container for determination of compliance. After 12 weeks the participants discontinued use of the product, and 12 weeks later they were retested.
The PFS is composed of 22 numerically scaled questions rated from 0 (no fatigue) to 10 (severe) fatigue. These items measure four dimensions of subjective fatigue: behavioral/severity (6 items); affective/meaning (5 items); sensory (5 items); and cognitive/mood (6 items). These are used to calculate the four sub-scale/dimensional scores and the total fatigue scores. The standardized alpha (Cronbach’s alpha) did not drop below 0.90 for any of the subscales, and the standard alpha for the entire scale of 22 questions was 0.96, indicating excellent reliability for an established instrument [14].
Mitochondrial function was determined by transport and reduction of the dye Rhodamine-123 as described previously [13]. Peripheral blood mononuclear cells (PBMC) were isolated from whole blood using a Ficoll-Hypaque gradient by centrifugation at 1,800 rpm for 30 min in a clinical centrifuge at room temperature. PBMC were stained with 2.0 or 10 mM of Rhodamine-123 (Sigma Chemical, St. Louis, MO) in phosphate-buffered saline (PBS) in the dark for 15 min at 370C. To remove unbound dye prior to flow cytometric analysis the cells were washed twice by centrifugation in ice-cold PBS and re-suspended in cold PBS. Non-viable cells were excluded from analysis using a light scatter gate established by staining with a propidium iodide (Sigma Chemical) solution. Data was collected on Rhodamine-123 fluorescence using an argon ion laser tuned at 488 nm (FACScan, Becton Dickinson, Mountain View, CA) and analyzed using CellQuest software (Becton Dickinson). Since the data using 2.0 or 10 mM of Rhodamine-123 were similar, only the data using the 10 mM dose was reported. Results from the mitochondrial staining with Rhodamine-123 were analyzed using a repeated measures analysis of variance (ANOVA) and Bonferroni/Dunn post-hoc test for specific group differences (young control, mild versus moderate fatigue, treatment times, washout, etc.).
RESULTS
NTF improved the overall fatigue scores of moderately fatigued subjects as measured by the PFS (Table 3). The initial PFS group average (mean) fatigue score was 5.75±0.6, and after four weeks of NTF this improved to 4.59±0.5 or a 20.2% reduction in fatigue. After eight and 12 weeks of NTF the PFS fatigue scores of the moderately fatigued group improved to 3.8±0.6 (33% reduction) and 3.71±0.65 (35.5% reduction), respectively (Table 3). These changes were significant (p<0.001). By sex, the total PFS mean score improved in moderately fatigued subjects after taking NTF for four weeks by 15.3% in women and 23.5% in men. After eight and 12 weeks, fatigue improved in women by 27.5% and 32.3%, respectively, and in men by 40.5% and 42.9%, respectively. There were no significant differences between the results with men and women. In contrast to moderately fatigued subjects, however, NTF use did not have a significant effect on mild fatigue. The improvement in fatigue scores overall after 12 weeks of NTF in mildly fatigued subjects was only 5.6% (Table 3). As found previously with severely fatigued subjects [12], age was not associated with the degree of change in fatigue in the moderately fatigued group using the NTF supplement. When subjects stopped using NTF, their fatigue scores increased. Twelve weeks after stopping NTF the moderately fatigued group had fatigue scores of 4.53±0.4 or 21.2% difference with the baseline value, whereas in the mildly fatigued group there were no significant differences in overall PFS fatigue scores (Table 3).
Using the PFS subscales the Behavioral/Severity scores improved in moderately fatigued subjects after 12 weeks of NTF an average of 15.3% (Table 3). The Affective/Meaning subscale improved by an average of 37% and 42.7% after NTF use for 8 and 12 weeks, respectively. The Sensory subscale revealed 37.9% and 40.5% average improvements for the group after 8 and 12 weeks NTF use, respectively. Finally, the Cognitive/Mood subscale showed average improvements of 37.2% and 32.9% after 8 and 12 weeks of NTF use, respectively (Table 3).
Mitochondrial function was measured in the various groups using the Rhodamine-123 assay. Since the data using 2 or 10 mM Rhodamine-123 were not significantly different, only the data using 10 mM dye was reported. The staining of mitochondria with Rhodamine-123 changed significantly throughout the course of treatment of both moderately and mildly fatigued subjects with NTF (Figures 1 and 2) (F [4,76]=29.917, p<0.0001). Post-hoc analysis with the Bonferroni/Dunn test for specific differences between groups indicated that after 8 and 12 weeks of NTF the results were significantly different from baseline (p<0.0001) and after washout for an additional 12 weeks (p<0.001); however, there was no significant difference between the use of NTF for 8 and 12 weeks (p>0.4). After 12 weeks of NTF the Rhodamine-123 mitochondrial assay yielded results similar to and not significantly different from those found in non-fatigued young adults that had not taken NTF (Figures 1 and 2). In the moderately fatigued group 8 or 12 weeks of NTF use resulted in significant differences (p<0.001) in mitochondrial function; however, there was no significant difference between the 8- and 12-week groups (p>0.17) (Figure 1). This amounted to an increase in mitochondrial function by 8.4%, 23.8% and 23.7%, respectively, after four, eight and 12 weeks of NTF use in moderately fatigued subjects (Figure 1). Some subjects were monitored 12 weeks after discontinuing use of NTF. Although still significantly different from baseline (p<0.001), mitochondrial function returned to intermediate values between baseline and the values at 8 or 12 weeks (Figures 1 and 2). When analyzed by sex, there were no significant differences between men and women in any of the groups.
DISCUSSION
Mitochondria are the most important source of cellular energy in our bodies. If their function is impaired, energy available to cells is limited to the Krebs Cycle. There are a number of conditions and substances that can impair mitochondrial function [5-8], but oxidation and damage of mitochodrial lipids in membranes are among the most important causes of impairment of mitochondrial function. This may result in modification of the electrical potential barrier across the mitochondrial membranes that is essential in the electron transport chain generation of cellular energy molecules. The dietary supplement NTF used in this pilot study is a unique mixture of cellular lipids that is rich in phospholipids and glycophospholipids, and in particular, polyunsaturated phosphatidylcholine and other membrane lipids. It also contains essential fatty acids and other lipids that are important in mitochondrial function and cellular membrane health and probiotic microorganisms to aid in intestinal uptake [12].
NTF has been used in clinical trials on cancer patients, and it has been shown to cause a substantial positive impact on fatigue. In a twelve week double-blinded, cross-over, placebo controlled, randomized trial on cancer patients receiving chemotherapy NTF in a vitamin-mineral supplement (PropaxTM) showed improvement from fatigue, nausea, diarrhea, impaired taste, constipation, insomnia and other quality of life indicators [10]. Most (64%) of the patients in the study reported significant improvement in these and other chemotherapy-induced side effects, and 29% experienced no overall worsening of side-effects. Following cross-over to the supplement containing NTF patients reported rapid improvement in nausea, impaired taste, tiredness, appetite, sick feeling and other indicators.
NTF has also demonstrated an anti-aging effect on hearing loss in aging rats. Using 18-20 month-old Harlan-Fisher 344 rats Seidman et al. [11] found that NTF prevented hearing loss associated with aging, shifting the threshold hearing from 35-40 dB in control aged animals to 13-17 dB in the test group. These results were found to be significant (p<0.005). They also found that NTF preserved cochlear mitochondrial function as measured in the Rhodamine-123 transport assay, increasing mitochondrial function by 34%. NTF also prevented the common aging-related mitochondrial DNA deletion (mtDNA4834) found in the cochlear of aging rats.
We also found an effect of PropaxTM with NTF in a pilot trial designed to measure fatigue in aged patients (>50 years-old) with a variety of common clinical conditions [12]. In these severely fatigued subjects (mean PFS scores = 7.9±0.82) NTF significantly reduced fatigue to moderate levels. After eight weeks of NTF there was a 40% reduction in overall fatigue (mean PFS scores = 4.7±2.01) as measured by the FPS instrument. These results are comparable to the data presented here for moderately fatigued subjects, where we found a 35.5% reduction in overall fatigue in moderately fatigued subjects after eight weeks use of NTF.
In the current study we utilized moderately (PFS scale=4-7) and mildly fatigued (PFS scale=1-4) subjects but only found a significant effect on fatigue in the moderately fatigued group of either sex. There could be a number of reasons for this observation, but it is unlikely that the only contribution to fatigue in these patients is mitochondrial function. Fatigue is a complex phenomenon, and it may be determined by several factors, including psychological health of the subjects. Also, in the mildly fatigued patients differences are difficult to determine because of the nature of the measuring instrument, and it might be unreasonable to expect significant differences in subjects that score very low initially on the PFS.
Our subjects were not randomly chosen for this study; they were recruited using a health talk radio program in the Los Angeles, CA region. The only criteria was that they were older than 50 years-old, mildly to moderately fatigued (using the PFS scales), and their fatigue could not be explained by an underlying clinical condition. Subjects were given a physical examination by a research nurse, and their blood was analyzed using a standard chemistry profile for possible clinical problems. This procedure did eliminate some prospective subjects from the trial. The subjects that qualified for the trial used NTF supplement for 12 weeks, and most of them then went off product for an additional 12 weeks to see if they would return to baseline fatigue and mitochondrial function values. Another potential problem was the number of participants with mild or moderate fatigue used in the study. Ideally, we would have liked to have a larger number of participants, but a number of factors prevented this, including the cost of the study.
Fatigue is related to the metabolic energy available to an individual and ultimately to the many cells that perform their myriad of functions. The integrity of cell and intracellular membrane structures, especially in the mitochondria, is critical to cell function and energy production [15-17]. NTF provides cells and mitochondria with the glycophospholipids, fatty acids and other essential lipids to repair and replace membrane components needed for maintenance of cell and mitochondrial function necessary in the production of cellular energy to combat fatigue.
The decline of energy production with aging may be due, in part, to mitochondrial lipid peroxidation by reactive oxygen species. Membrane damage and subsequent mitochondrial dysfunction can also lead to modifications (especially mutations and deletions) in mitochondrial DNA (mtDNA). The mitochondrial theory of aging proposes that the development of chronic degenerative diseases is the result, in part, of accumulated mtDNA mutations and deletions and oxidative damage to mitochondrial membranes over time. Indeed, some studies have linked the development of certain chronic diseases with the degree of mitochondrial membrane lipid peroxidation and mtDNA damage. Thus the damage to mtDNA and mitochondrial membranes seems to be involved in the etiology of age-associated degenerative diseases leading to changes in the expression of genes important for cell survival as well as the phenomenon of aging itself [18]. Restoration of mitochondrial membrane integrity and fluidity are essential for the optimal functioning of the electron transport chain [19]. Declines in energy production with aging coupled with an increase in oxidative stress can modify membrane lipids and increase mitochondrial membrane permeability and activate cellular death programs (apoptosis). Together these factors likely play a major role in the aging process and they also affect the development of age-related degenerative diseases [20].
The first outward sign of cellular deterioration may be fatigue. As the phospholipid structure of the mitochondrial membrane loses fluidity and becomes more porous at lipid/protein interfaces in the membrane, the membrane potential is affected and less able to maintain the electron transport process. In addition, the electron transport chain increases the production of Reactive Oxygen Species (ROS), free radicals that can further damage mitochondrial membranes and mtDNA. Although there is always some inherent mitochondrial membrane leakage and damage, this is usually repaired, unless the rate of repair is exceeded by the rate of oxidative damage [21].
Finally, since ROS are highly implicated in age-associated mtDNA damage, we tried to determine the age-dependent accumulation of a particular 4977 bp mtDNA deletion in platelets of patients. The 83 bp fragment of deleted mtDNA was detected in all samples including control blood from young volunteers. The amount of the deletion in blood cells did not show an age-dependent increase, and differences in amounts (quantity was estimated by fluorescence intensity of ethidium bromide stained DNA in agarose gel) of deletion were detected in blood before and after NTF use (data not shown). The 4977 bp deletion in mtDNA is known to accumulate with age in various human postmitotic tissues, such as brain, heart and skeletal muscle. Different groups have tried to use blood cells as a possible model for screening the accumulation of mtDNA mutations; however, the results have so far been contradictory. Biagini et al. [22] as well as the other groups [23-26] failed to detect this particular deletion in blood or platelets both from young and old individuals, whereas Meissner et al. [23] demonstrated that this deletion is detectable in blood cells, but the amount is substantially lower than in postmitotic tissues. Also Meissner et al. [23] did not show an age-dependent increase in the 4977 bp deletion in mtDNA. Our data are in good accordance with the assertion of Meissner et al. [23] that the accumulation of 4977 bp mtDNA deletion in blood cells is not age-dependent, and this might be explained by higher turnover rate of blood cells.
Acknowledgments
We acknowledge the excellent assistance of Christy Bennet and Ned Realiza. This study was supported by a grant from Nutritional Therapeutics, Inc. Dr. Berns was previously a consultant for Nutritional Therapeutics, Inc.
References
1. Kroenke K, Wood DR, Mangelsdorff AD, Meier NJ, Powell JB. Chronic fatigue in primary care. Prevalence, patient characteristics, and outcome. JAMA. 1988; 260: 929-934.
2. Morrison JD. Fatigue as a presenting complaint in family practice. J Family Pract 1980; 10: 795-801.
3. McDonald E, David AS, Pelosi AJ, Mann AH. Chronic fatigue in primary care attendees. Psychol Med. 1993; 23: 987-998
4. Piper BF, Linsey AM, Dodd MJ. Fatigue mechanism in cancer. Oncol Nursing Forum. 1987; 14: 17-23.
5. Richter C, Par JW, Ames B. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc Nat Acad Sci. USA 1998; 85: 6465-6467.
6. Wei YH, Lee HC. Oxidative stress, mitochondrial DNA mutation and impairment of antioxidant enzymes in aging. Exp Biol Med. 2002; 227:671-682.
7. Spector AA, Yorek MA. 1985. Membrane Lipid composition and cellular function. J Lipid Res. 1985; 26:10105.
8. Harman D. Aging: A theory based on free radical and radiation chemistry. J Gerontol. 1956; 2: 298-300.
9. Xu D, Finkel T. A role for mitochondria as potential regulators of cellular life span. Biochem Biophysics Res Commun 2002; 294:245-248.
10. Colodny L, Lynch K, Farber C, Papish S, et al. Results of a study to evaluate the use of Propax to reduce adverse effects of chemotherapy. JANA 2000; 2: 17-25.
11. Seidman M, Khan MJ, Tang WX, Quirk WS. Influence of lecithin on mitochondrial DNA and age-related hearing loss. Otolaryngol Head Neck Surg 2002; 127:138-144.
12. Ellithorpe RR, Settineri R, Nicolson GL. Pilot Study: Reduction of fatigue by use of a dietary supplement containing glycophospholipids. JANA 2003; in press.
13. Kim MJ, Cooper DD, Hayes SF, Spangrude GJ. Rhodamine-123 staining in hematopoietic stem cells of young mice indicates mitochondrial activation rather than dye efflux. Blood 1998; 91: 4106-4117.
14. Nunnally JC. 1978. Psychometric Theory (2nd ed.) New York: McGraw-Hill, pp. 117-123.
15. Zeisel SH, In Hanin I, Popell G, (eds.) 1996. Phospholipids, biochemical pharmaceutical and analytical considerations. New York: Plenum Press, pp. 219-231.
16. Conlay LA, Wurtman RJ, Blusztajn K, Coviella IL, Maher TJ, Evoniak GE. N Engl J Med. 1986; 175: 892.
17. Johns DR. 1995. Seminars in medicine of Beth Israel Hospital, Boston: Mitochondrial DNA and Disease. N Engl J Med. 1995; 333: 638-44.
18. Kowald A. The mitochondrial theory of aging: do damaged mitochondria accumulate by delayed degradation? Exp Gerontol 1999; 34:605-612.
19. Paradies G, Petrosillo G, Pistolese M, Ruggiero F. Reactive oxygen species affect mitochondrial electron transport complex I activity through oxidative cardiolipin damage. Gene 2002; 286:135-141.
20. Lin M, Simon D, Ahn C, Lauren K, Beal MF. High aggregrate burden of somatic mtDNA point mutations in aging and Alzheimer’s disease brain. Human Mol Genet 2002; 11:133-145.
21. Koboska J, Coskun P, Esposito L, Wallace DC. Increased mitochondrial oxidative stress in the Sod2(+/-) mouse results in age-related decline of mitochondrial function culminating in increased apoptosis. Proc Nat Acad Sci USA 2001; 98:2278-2283.
22. Biagini G, Pallotti F, Carraro S, Sgarbi G, Pich MM, Lenaz G, Anzivino F, Gualandi G, Xin D.
Mitochondrial DNA in platelets from aged subjects. Mech Ageing Dev 1998; 101:269-275.
23. Meissner C, Mohamed SA, Klueter H, Hamann K, von Wurmb N, Oehmichen M. Quantification of mitochondrial DNA in human blood cells using an automated detection system. Forensic Sci Int 2000; 113:109-112.
24. Lee HC, Oang CY, Hsu HS, Wei YH. Deletion in blood mitochondrial DNA in Kearns-Sayre syndrome. Biochim Biophys Acta 1994; 1226:37-43.
25. Poulton J, Deadman ME, Ramacharan S, Gardiner RM. Germ-line deletions of mtDNA in mitochondrial myopathy. Am J Hum Genet 1991; 90:649-653.
26. Smith OP, Hamm MJ, Woodward CE, Brockington M. Pearson’s marrow/pancreas syndrome: haematological features associated with deletion and duplication of mitochondrial DNA. Br J Haemotol 1995; 90:469-472.
OPTI Factor and Chronic Fatigue
OPTI Factor contains NT Factor® daily which is the most powerful proprietary substance to combat aging and fatigue.
Lipid replacement therapy (LRT) has been used along with other strategies, such as antioxidant therapy, to replace damaged or oxidized cellular lipids that accumulate during aging and in various clinical conditions. Differing from traditional lipid nutritional supplementation, LTR replacement lipids are protected from oxidation and damage during storage, ingestion and digestion. Important lipids that require constant replacement are phospholipids, glycophospholipids and other lipids that make up cellular and organelle membranes, especially mitochondrial membranes. Decreased mitochondrial function and loss in the efficiency of the electron transport chain are related to aging and fatigue. Oxidative damage to mitochondria, mainly from Reactive Oxygen Species (ROS), results in peroxidation of cellular and mitochondrial lipids, proteins and DNA, but it is ROS damage to mitochondrial membrane lipids that may cause the most rapid loss of mitochondrial function. LRT along with antioxidants can circumvent ROS membrane damage and replace and restore mitochondrial and other cellular membrane functions via delivery of replacement lipids in their unoxidized, undamaged states. Recent clinical trials have shown the benefit of LRT plus antioxidants in restoring mitochondrial electron transport function and reducing fatigue. In aging subjects mitochondrial function was restored to levels found in young adults in consort with reductions in fatigue, suggesting the anti-aging and anti-fatigue benefits of LRT plus antioxidants in protecting mitochondrial and other cellular membranes from oxidative and other damage and preventing loss of function.
The use of natural lipids for dietary support and even therapy for various medical conditions has a long and rich history and will not be dealt with in this brief commentary. Instead I will concentrate on discussing recent clinical trials that have shown the effectiveness of lipid replacement therapy (LRT) plus antioxidants in the treatment of certain clinical disorders and conditions as well its use in anti-aging supplements. LRT is not just the dietary substitution of certain lipids with proposed health benefits; it is the actual replacement of damaged cellular lipids with undamaged lipids to ensure proper structure and function of cellular structures, mainly cellular and organelle membranes. This constitutes the most important functional use of lipids in our bodies. Damage to membrane lipids can impair fluidity, electrical properties, enzymatic activities and transport functions of cellular and organelle membranes.1-3
An important difference between simple lipid dietary supplementation and LRT is that the lipids in LRT must be protected from oxidative and other damage during storage and during the ingestion, digestion and absorption processes in vivo. Thus LRT should result in delivery of high concentrations of unoxidized, undamaged lipids, and this is important in reversing the damage and restoring function to (partially oxidized) cellular membranes. Combined with antioxidant supplements, LTR has proven to be an effective method to prevent aging-associated changes in certain cellular activities and functions and for use in the treatment of certain clinical conditions.
n-3 LIPID SUPPLEMENTS AND CHRONIC ILLNESSES
In the past several years different sources of lipid dietary mixtures have been used to improve general health or for more specific uses, such as in the treatment of cardiovascular diseases and inflammatory disorders.4-10 Although not every clinical study has found health benefits from supplementing specific lipids in the diet,7,11 most studies have documented the value of dietary supplements that favor certain types of lipids over others. The most common substitution is the dietary administration of lipids where n-3 polyunsaturated fatty acids (mainly fish- or flaxseed-derived) are favored relative to n-6 lipids.4-10
Oral administration of n-3 polyunsaturated fatty acids has been beneficial in various clinical conditions. This includes reduction in risk of coronary heart disease11-14 and death due to cardiac arrest,15-17 age-associated macular degeneration,18 asthma,19 ulcerative colitis,20,21 Crohn’s disease,22 IgA nephropathy,23,24 rheumatoid arthritis,25,26 diabetes mellitus,27,28 various malignancies29,30 and other conditions. Discrepancies and conflicting results in some clinical studies on the health benefits of n-3 polyunsaturated fatty acids could be the result of insufficient care in the storage, preservation, dose and administration of the dietary lipid mixtures.31
INGESTED LIPIDS ARE QUICKLY ADSORBED AND TRANSPORTED TO TISSUES
Lipids such as those found in various cellular compartments are in dynamic equilibrium in the body, and this is why LRT is possible. Orally ingested lipids diffuse to the gut epithelium and are bound and eventually transported into the blood and lymph using specific (carrier alipoproteins) and nonspecific (partitioning and diffusion) mechanisms.32-34 Within minutes, lipid molecules are transported from gut epithelial cells to endothelial cells, then excreted into and transported in the circulation bound to lipoproteins and blood cells.34,35 Once in the circulation, specific lipoprotein carriers and red blood cells protect lipids throughout their passage and eventual deposition onto specific cell membrane receptors where they can be taken into cells via endosomes and by diffusion.36 Inside the cells, lipid transporters deliver specific lipids to cell organelles where they are taken in by specific transport proteins and by partitioning and diffusion.37 Once undamaged lipids such as phosphotidylethanolamine are transported to mitochondria, they can be used to synthesize other lipids, such as phosphotidylserine. This system works efficiently, probably due to the concentration gradients that exist from the gut during the digestion of lipids to their absorption by gut epithelial cells and their transfer to the blood, to the tissues, and ultimately to the cells’ interior. Damaged lipids can be removed by a similar reverse process that may be driven by lipid transfer proteins and by enzymes that recognize and degrade damaged lipids.38
FATIGUE, AGING AND OXIDATIVE DAMAGE TO MITOCHONDRIA
Many medical conditions are associated with fatigue, including respiratory, coronary, musculoskeletal, and bowel conditions as well as various cancers and infections.39,40 Chronic fatigue (intractable fatigue lasting more than 6 months that is not reversed by sleep) is the most common complaint of patients seeking medical care.41,42 It is an important secondary condition in many clinical diagnoses, often preceding and is related to patients’ diagnoses.42,43 The phenomenon of fatigue has only recently been defined as a multidimensional sensation, and attempts have been made to determine the extent of fatigue and its possible causes.40,43 Most patients understand fatigue as a loss of energy and inability to perform even simple tasks without exertion. Using the Piper Fatigue Scale measurement tool that combines multiple fatigue-associated elements into an overall score fatigue has been quantitated as a multi-component sensation.40,43 We have successfully used the Piper Fatigue Scale in clinical studies on aging subjects who complain of fatigue to determine their responses to LRT plus antioxidants.44,45
The complex phenomenon called fatigue is involved with cellular energy systems found primarily in the mitochondria. Damage to cellular mitochondria can impair the abilities of cells to produce high-energy molecules, such as ATP and NADH. This occurs naturally with aging and during chronic illness, mainly by the build up of damaged mitochondrial components that impair function. During aging the production of Reactive Oxygen Species (ROS), made up of oxidative and free radical oxygen- and nitrogen-containing molecules, such as nitric oxide, oxygen and hydroxide radicals and other molecules, can cause oxidative stress and cellular damage, resulting in oxidation of lipids, proteins (enzymes) and DNA. Once oxidized, these cellular molecules are structurally and sometimes functionally changed. Major targets of cellular ROS damage are mitochondria and nuclei, mainly their phospholipid/protein membranes and DNA.3,46-49 Damage to the former results in alterations in membrane fluidity and electrical properties, whereas damage to protein enzymes and deletions or modifications in DNA structure can result in alterations in enzyme activities and gene expression.
Mitochondria themselves produce some ROS as a consequence of oxidative phosphorylation,50 but excess ROS production throughout our lifetimes can result in accumulation of mitochondrial and nuclear damage. To counter this, cellular free-radical-scavenging enzymes neutralize excess ROS and repair enzymes reverse ROS-mediated damage.50 Although some ROS production is important in triggering cell proliferation, gene expression and destruction of invading microbes,51 with aging, ROS damage accumulates because antioxidant enzymes and enzyme repair mechanisms along with biosynthesis cannot restore or replace enough ROS-damaged molecules.3,46,47 Disease and infection can also result in similar damage that exceeds the abilities of cellular systems to neutralize, repair, or replace damaged molecules.3,50
Mitochondria from aging animals show higher levels of accumulated ROS damage to mitochondrial membranes, enzymes and DNA than found in young animals.3,51 At the molecular level, damage to phospholipids and other lipids in mitochondrial membranes by ROS free-radicals can affect membrane integrity, fluidity and transmembrane electrical potentials, resulting in damage to the electron transport chain and its associated components and loss of function.3,50 Young cells and organisms can cope with ROS since they possess high levels of free-radical scavenging systems that neutralize ROS, such as superoxide dismutase and glutathione reductase. They also have a higher capacity to repair or replace damage caused by ROS. With aging these homeostatic systems naturally decline and can be overwhelmed by ROS and oxidative stress.51,52 Since the aging process results in mitochondria accumulating ROS damage to their membranes, enzymes and DNA, this is thought to contribute to or even be a cause of the aging process.3,47,51-53
MANAGING ROS-MEDIATED DAMAGE WITH ANTIOXIDANTS
Reducing cellular and mitochondrial membrane and DNA damage and loss of membrane integrity are important in preventing loss of cellular energy and regulating cellular life span.3,54 This can be done, in part, by neutralizing ROS with various antioxidants or increasing free-radical scavenging systems that neutralize ROS. Dietary antioxidants and some accessory molecules, such as zinc and certain vitamins, are important in maintaining free-radical scavenging systems, biosynthetic capacity, membranes, enzymes and DNA. There are at least 40 micronutrients required in the human diet,55 and aging increases the need to supplement these in a normal diet to prevent age-associated declines in mitochondrial and other cellular functions. Although very important, antioxidant use alone may not be sufficient to maintain cellular components free of ROS damage. This is why LRT is important in replacing ROS-damaged lipids along with antioxidant use to prevent further oxidation.
In animal studies the effects of reducing ROS have been dramatic in aging and disease models. For example, in rodents there are age-dependent losses in antioxidants and antioxidant vitamins as well as reductions in glutathione and levels of antioxidant enzymes.56 In an aged rat study, the effects of alpha-lipoic acid and other dietary antioxidants on the levels of cellular antioxidants, such as reduced glutathione and vitamins C and E, levels of mitochondrial membrane lipid peroxidation and activities of mitochondrial electron transport and accessory enzymes, have been investigated and found to decrease but not eliminate ROS damage to the electron transport chain.57 Thus dietary antioxidant supplementation partially reversed the age-related declines in cellular antioxidants and mitochondrial enzyme activities and prevented mitochondria from most age-associated functional decline. In another study rats were fed diets supplemented with coenzyme Q10, alpha-lipoic acid, melatonin, or alpha-tocopherol for a six-month period. They found that the antioxidant mixture could inhibit the progression of certain age-associated changes in cerebral mitochondrial electron transport chain enzyme activities.58,59 Thus animal studies have shown that antioxidants can prevent, at least in part, age-associated changes in mitochondrial structure and function. However, antioxidants alone cannot completely eliminate ROS damage to mitochondria, and this is why LRT is an important adjunct to antioxidant administration.
In addition to the aging-associated oxidative changes in mitochondrial enzymes and lipids, mitochondrial DNA also accumulates oxidative damage during the aging process.3,51-54,60,61 To prevent this, antioxidants have also been useful, such as vitamins C and E, coenzyme Q10, sulfur-containing antioxidants and plant antioxidant extracts.62,63 Age-associated damage to mitochondrial DNA may affect their ability to function due, in part, to a loss in ability to synthesize and replace critical mitochondrial enzymes.
Antioxidants may also affect the pathogenic processes of certain diseases.50,60 The experimental dietary use of antioxidants can prevent age-associated mitochondrial dysfunction and damage, inhibit the age-associated decline in immune and other functions and prolong the lifespan of laboratory animals.3,56-59,63,64
ANIMAL STUDIES USING LIPID REPLACEMENT THERAPY AND ANTIOXIDANTS
Another method used to reverse damage to tissue membranes is to replace damaged cellular and mitochondrial membrane phospholipids and other lipids using dietary supplements containing polyunsaturated phosphatidylcholines and other phospholipids, glycophospholipids and fatty acids that are essential structural and functional components of all biological membranes.44,45 One such LRT dietary supplement is called NT Factor,™ and it has been used successfully in animal and clinical lipid replacement studies. Its encapsulated lipids are protected from oxidation in the gut by the inclusion of antioxidants and can be absorbed and transported into tissues without undue damage.44,45 NT Factor contains a variety of components (Table 1), including glycophospholipids and other lipids, antioxidants, nutrients, probiotics, vitamins, minerals and plant extracts.44
NT Factor has been used to produce an anti-aging effect in aged laboratory animals. In 18- to 20-month-old rats, Seidman et al65 found that NT Factor prevented hearing loss associated with aging and shifted the threshold hearing from 35-40 dB in control aged animals to 13-17 dB in the NT Factor group. These results were highly significant (p<0.005). They also found that NT Factor preserved cochlear mitochondrial function as measured in a Rhodamine-123 transport assay, increasing mitochondrial function by 34%. In these experiments, Rhodamine-123 is transported into mitochondria where it is chemically reduced to its fluorescent form only under conditions where mitochondria are fully functional.66 NT Factor also prevented a common aging-related mitochondrial DNA deletion (mtDNA4834) found in the cochlear of aging rats.65 Thus LRT plus antioxidants was successful in preventing age-associated hearing loss and mitochondrial damage in an animal model for aging.
CLINICAL STUDIES USING LIPID REPLACEMENT THERAPY AND ANTIOXIDANTS
LRT plus antioxidants has been successfully used in clinical studies to reduce fatigue and protect cellular and mitochondrial membranes from damage by ROS. For example, NT Factor has been used in a vitamin and mineral mixture (Propax™) in cancer patients to reduce the effects of cancer therapy, such as chemotherapy-induced fatigue, nausea, vomiting, and other side effects associated with chemotherapy.67 In a twelve-week double-blinded, cross-over, placebo controlled, randomized trial on cancer patients receiving chemotherapy, Propax supplementation resulted in improvement from fatigue, nausea, diarrhea, impaired taste, constipation, insomnia and other quality of life indicators.67 The majority (64%) of the patients in this study reported significant reductions in chemotherapy-induced side effects, and 29% experienced no overall worsening of chemotherapy side-effects. Following cross-over to the supplement containing the Propax, patients reported rapid improvement in nausea, impaired taste, tiredness, appetite, sick feeling and other indicators associated with chemotherapy.67
We have used Propax plus NT Factor in an LRT study with severely fatigued, aged subjects (>60 years-old) with a variety of clinical diagnoses to reduce fatigue, as measured by the Piper Fatigue Scale.40,43 We found that fatigue was reduced approximately 40%, from severe to moderate fatigue, after eight weeks of using Propax containing NT Factor. The results were highly significant (p<0.0001).45 A more recent LRT plus antioxidant study was initiated to examine the effects of NT Factor on fatigue in moderately and mildly fatigued subjects and to determine if their mitochondrial function, as measured by the transport and reduction of Rhodamine-123, and fatigue scores improved with administration of NT Factor.44 Using NT Factor for eight or twelve weeks resulted in a 33% or 35.5% reduction in fatigue, respectively. The results were highly significant (p<0.001) and were obtained using the Piper Fatigue Scale for measuring fatigue.44
In the LRT/antioxidant trial with moderately fatigued patients, reductions in fatigue paralleled significant gains in mitochondrial function.44 In fact, there was good correspondence between reductions in fatigue and gains in mitochondrial function. After only eight weeks of NT Factor, mitochondrial function was significantly improved (p<0.001). Interestingly, after twelve weeks of NT Factor use, mitochondrial function was found to be similar to that of young, healthy adults.44 After twelve weeks of NT Factor use, subjects discontinued the supplement for an additional twelve weeks, when their fatigue and mitochondrial function were again measured. After the twelve-week wash-out period, fatigue and mitochondrial function were intermediate between the initial starting values and those found after eight or twelve weeks, indicating that continued use of the supplement is probably required to maintain lower fatigue scores and show improvements in mitochondrial function.44 The results indicate that LRT/antioxidants can significantly improve and even restore mitochondrial function and improve fatigue scores in aging human subjects.
CHRONIC FATIGUE, MITOCHONDRIAL FUNCTION AND DEGENERATIVE DISEASE
When mitochondrial function is impaired, the net energy available to cells is limited to the Krebs Cycle and anaerobic metabolism. There are a number of conditions and substances that can impair mitochondrial function,45,46,54 but oxidation and damage of mitochondrial lipids in membranes are thought to be among the most important causes.3,54,68 Oxidation of membrane lipids results in modification of membrane fluidity and the electrical potential barrier across mitochondrial membranes, essential elements in the proper functioning of the electron transport chain.3,54,68 Mitochondrial function appears to be directly related to fatigue, and when patients experience fatigue their mitochondrial function is inevitably impaired. Fatigue is a complex phenomenon determined by several factors, including psychological health. At the biochemical level fatigue is related to the metabolic energy available to tissues and cells. Thus the integrity of cellular and intracellular membranes, especially in the mitochondria, is critical to cell function and energy metabolism. When mitochondrial membrane glycophospholipids, phospholipids, fatty acids, and other essential lipids are damaged by oxidation, they must be repaired or replaced in order to maintain the production of cellular energy to alleviate fatigue.
The decline of cellular energy production with aging appears to be due, in part, to mitochondrial lipid peroxidation by ROS and the failure to repair or replace damaged molecules at a rate that exceeds their damage. Membrane damage and subsequent mitochondrial dysfunction by ROS can also lead to modifications (especially mutations and deletions) in mitochondrial DNA (mtDNA). The mitochondrial theory of aging proposes that the development of chronic degenerative diseases is the result, in part, of accumulated mtDNA mutations and deletions and oxidative damage to mitochondrial membranes over time.3,54,61,68,69 Indeed, these studies have linked the development of certain chronic diseases with the degree of mitochondrial membrane lipid peroxidation and mtDNA damage. Thus the damage to mtDNA and mitochondrial membranes seems to be involved in the etiology of age-associated degenerative diseases leading to changes in the expression of genes important for cell survival as well as those that control aging.69 Restoration of mitochondrial membrane integrity, fluidity and other properties are essential for the optimal functioning of the electron transport chain and oxidative generation of ATP and NADH. Declines in energy production with aging and disease coupled with increases in oxidative stress can change gene expression programs and activate cellular apoptosis programs.70 Apoptosis can also be attenuated with the administration of n-3 polyunsaturated fatty acids.71
The ability to control membrane lipid peroxidation and DNA damage likely play a major role in the aging process and the development of age-related degenerative diseases.3,60,72 LRT has proven to be a valuable tool in helping maintain mitochondrial function, and along with combined antioxidant use LRT should be an important part of anti-aging strategies as well as strategies used to treat various age-associated degenerative diseases and conditions.
1. Nicolson GL, Poste G, Ji T. Dynamic aspects of cell membrane organization. Cell Surface Rev. 1977;3:1-73.
2. Subczynski WK, Wisniewska A. Physical properties of lipid bilayer membranes: relevance to membrane biological functions. Acta Biochim Pol. 2000;47:613-625.
3. Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Nat Acad Sci USA. 1994;91:10771-10778.
4. Harris WS. n-3 fatty acids and lipoproteins: comparison of results from human and animal studies. Lipids. 1996;31:243-252.
5. Connor WE. Importance of n-3 fatty acids in health and disease. Am J Clin Nutr. 2000;71:S171-S178.
6. Nordoy A, Marchioli R, Arnesen H, Videback J. n-3 polyunsaturated fatty acids and cardiovascular diseases. Lipids. 2001;36:S127-S129.
7. Butcher G, Hengstler HC, Schindler P, Meier C. n-3 polyunsaturated fatty acids in coronary heart disease: a meta-analysis of randomized controlled trials. Am J Med. 2002;112:298-304.
8. Belluzzi A. n-3 fatty acids for the treatment of inflammatory bowel diseases. Proc Nutr Soc. 2002; 61:391-393.
9. Calder PC. Dietary modification of inflammation with lipids. Proc Nutr Soc. 2002; 61:345-358.
10. Grimble RF. Nutritional modulation of immune function. Proc Nutr Soc. 2001;60:389-397.
11. Angerer C, Stork W, von Schacky S. Effect of dietary supplementation with omega-3 fatty acids on progression of artherosclerosis in carotid arteries. Cardiovasc Res. 2002;54:183-190.
12. Schmidt J, Skou EB, Christensen HA, Dyerberg JH. N-3 fatty acids from fish and coronary artery disease: implications for public health. Public Health Nutr. 2000;3(1):91-98.
13. Hu JE, Bronner FB, Willett L, Stampfer WC, Rexrode MJ, et al. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA. 2002;287:1815-1821.
14. Kinsella RA, Lokesh JE, Stone B. Dietary n-3 polyunsaturated fatty acids and amelioration of cardiovascular disease: possible mechanisms. Am J Clin Nutr. 1990;52:1-28.
15. Siscovick LH, Raghunathan DS, King TE, Weinmann I, et al. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. JAMA. 1995; 274:1363-1367.
16. Hu WC, Manson FB, Willett JE. Types of dietary fat and risk of coronary heart disease: a critical review. J Am Coll Nutr. 2001;20:5-19.
17. Bucher G, Hengstler HC, Schindler P, Meier C. N-3 polyunsaturated fatty acids in coronary heart disease: a meta-analysis of randomized controlled trials. Am J Med. 2002;112:298-304.
18.Seddon W, Rosner JM, Sperduto B, Yannuzzi RD, et al. Dietary fat and risk for advanced age-related macular degeneration. Arch Ophthalmol. 2001;119:1191-1199.
19. Peat JK. Prevention of asthma. Eur Respir J. 1996;9:1545-1555.
20. Aslan G, Triadafilopoulos A. Fish oil fatty acid supplementation in active ulcerative colitis: a double-blind, placebo-controlled, crossover study. Am J Gastroenterol. 1992; 87:432-437.
21. Stenson W, Cort WF, Rodgers D, Burakoff J, DeSchryver Kecskemeti R, et al. Dietary supplementation with fish oil in ulcerative colitis. Ann Intern Med. 1992; 116: 609-614.
22. Belluzzi M, Brignola A, Campieri C, Pera M, et al. Effect of an enteric-coated fish-oil preparation on relapses in Crohn’s disease. N Engl J Med. 1996; 334:1557-1560.
23. Donadio JP, Larson Jr JV, Bergstralh TS, Grande EJ, et al. A randomized trial of high-dose compared with low-dose omega-3 fatty acids in severe IgA nephropathy. J Am Soc Nephrol. 2001; 12:791-799.
24. Donadio KE, Bergstralh JV, Offord EJ, Spencer KP, et al. A controlled trial of fish oil in IgA nephropathy. Mayo Nephrology Collaborative Group. N Engl J Med. 1994;331:1194-1199.
25. Kremer JM. Effects of modulation of inflammatory and immune parameters in patients with rheumatic and inflammatory disease receiving dietary supplementation of n-3 and n-6 fatty acids. Lipids. 1996;31:S243-S247.
26. Ariza MH, Mestanza Peralta R, Cardiel M. Omega-3 fatty acids in rheumatoid arthritis: an overview. Semin Arthritis Rheum. 1998;27:366-370.
27. Malasanos PW, Stacpoole TH. Biological effects of omega-3 fatty acids in diabetes mellitus. Diabetes Care. 1991;14:1160-1179.
28. Landgraf Leurs R, Drummer MM, Froschl C, Steinhuber H, et al. Pilot study on omega-3 fatty acids in type I diabetes mellitus. Diabetes. 1990;39:369-375.
29. Gogos F, Ginopoulos CA, Salsa P, Apostolidou B, et al. Dietary omega-3 polyunsaturated fatty acids plus vitamin E restore immunodeficiency and prolong survival for severely ill patients with generalized malignancy: a randomized control trial. Cancer. 1998;82:395-402.
30. Daly M, Weintraub JM, Shou FN, Rosato J, Lucia EF. Enteral nutrition during multimodality therapy in upper gastrointestinal cancer patients. Ann Surg. 1995;221:327-338.
31. Takahata PC, Monobe K, Tada K, Weber M. The benefits and risks of n-3 polyunsaturated fatty acids. Biosci Biotechnol Biochem. 1998;62:2079-2085.
32. Hajri T, Abumrad NA. Fatty acid transport across membranes: relevance to nutrition and metabolic pathology. Annu Rev Nutr. 2002; 22:383-415.
33. Schmitz G, Langmann T, Heimerl S. Role of ABCG1 and other ABCG family members in lipid metabolism. J Lipid Res. 2001;42:1513-1520.
34. Hamilton JA. Fatty acid transport: difficult or easy? J Lipid Res. 1998;39(3):467-481.
35.Fellmann P, Herve P, Pomorski T, Muller P, et al. Transmembrane movement of diether phospholipids in human erythrocytes and human fibroblasts. Biochemistry. 2000;39:4994-5003.
36. Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature 2003;422:37-44.
37. Mansbach CM, Dowell R. Effect of increasing lipid loads on the ability of the endoplasmic reticulum to transport lipid to the Golgi. J Lipid Res. 2000;41: 605-612.
38. E. Bruce C, Chouinard RA, Tall AR. Plasma lipid transfer proteins, high-density lipoproteins, and reverse cholesterol transport. Annu Rev Nutr. 1998;18:297-330.
39. McDonald E, David AS, Pelosi AJ, Mann AH. Chronic fatigue in primary care attendees. Psychol Med. 1993;23:987-998.
40. Piper BF, Linsey AM, Dodd MJ. Fatigue mechanism in cancer. Oncol Nursing Forum. 1987; 14:17-23.
41. Kroenke K, Wood DR, Mangelsdorff AD, et al. Chronic fatigue in primary care. Prevalence, patient characteristics, and outcome. JAMA. 1988;260:929-934.
42. Morrison JD. Fatigue as a presenting complaint in family practice. J Family Pract. 1980;10:795-801.
43. Piper BF, Dribble SL, Dodd MJ, et al. The revised Piper Fatigue Scale: psychometric evaluation in women with breast cancer. Oncol Nursing Forum. 1998;25:667-684.
44. Agadjanyan M, Vasilevko V, Ghochikyan, et al. Nutritional supplement (NT Factor) restores mitochondrial function and reduces moderately severe fatigue in aged subjects. J Chronic Fatigue Syndr. 2003; 11(4):in press.
45. Ellithorpe RR, Settineri R, Nicolson GL. Pilot study: reduction of fatigue by use of a dietary supplement containing glycophospholipids. JANA. 2003;6(1):23-28.
46. Richter C, Par JW, Ames B. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc Nat Acad Sci USA. 1998; 85:6465-6467.
47. Wei YH, Lee HC. Oxidative stress, mitochondrial DNA mutation and impairment of antioxidant enzymes in aging. Exp Biol Med. 2002;227:671-682.
48. Spector AA, Yorek MA. 1985. Membrane lipid composition and cellular function. J Lipid Res. 1985;26:101-105.
49. Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956; 2:298-300.
50. Halliwell B. Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging. 2001;18:685-716.
51. Chen D, Cao G, Hastings T, et al. Age-dependent decline of DNA repair activity for oxidative lesions in rat brain mitochondria. J Neurochem. 2002;81:1273-1284.
52. Oslewacz HD. Genes, mitochondria and aging in filamentous fungi. Ageing Res Rev. 2002; 1:425-442.
53.Barja G. Endogenous oxidative stress: relationship to aging, longevity and caloric restriction. Ageing Res Rev. 2002;1:397-411.
54. Xu D, Finkel T. A role for mitochondria as potential regulators of cellular life span. Biochem Biophysi Res Commun. 2002;294:245-248.
55. Ames BM. Micronutrients prevent cancer and delay aging. Toxicol Lett. 1998;102:1035-1038.
56. De AK, Darad R. Age-associated changes in antioxidants and antioxidative enzymes in rats. Mech Ageing Dev. 1991;59:123-128.
57. Arivazhagan P, Ramanathan K, Panneerselvam C . Effect of DL-alpha-lipoic acid on mitochondrial enzymes in aged rats. Chem Biol Interact. 2001; 138:189-198.
58. Sharman EH, Bondy SC. Effects of age and dietary antioxidants on cerebral electron transport chain activity. Neurobiol Aging. 2001;22:629-634.
59. Sugiyama S, Yamada K, Ozawa T. Preservation of mitochondrial respiratory function by coenzyme Q10 in aged rat skeletal muscle. Biochem Mol Biol Int. 1995;37:1111-1120.
60. Lin M, Simon D, Ahn C, Lauren K, Beal MF. High aggregrate burden of somatic mtDNA point mutations in aging and Alzheimer’s disease brain. Human Mol Genet. 2002;11:133-145.
61. Sastre J, Pallardo FV, Garcia de la Asuncion J, Vina J. Mitochondria, oxidative stress and aging. Free Radical Res. 2000;32(3):189-198.
62. Kagan T, Davis C, Lin L, Zakeri Z. Coenzyme Q10 can in some circumstances block apoptosis, and this effect is mediated through mitochondria. Ann NY Acad Sci. 1999;887:31-47.
63. Matthews RT, Yang L, Browne S, et al. Coenzyme Q10 administration increases brain mitochondrial concentrations and exerts neuroprotective effects. Proc Natl Acad Sci USA. 1998;95:8892-8897.
64. Miquel, J. Can antioxidant diet supplementation protect against age-related mitochondrial damage? Ann NY Acad Sci. 2002; 959:317-347.
65. Seidman M, Khan MJ, Tang WX, Quirk WS. Influence of lecithin on mitochondrial DNA and age-related hearing loss. Otolaryngol Head Neck Surg. 2002;127:138-144.
66. Kim MJ, Cooper DD, Hayes SF, Spangrude GJ. Rhodamine-123 staining in hematopoietic stem cells of young mice indicates mitochondrial activation rather than dye efflux. Blood. 1998;91:4106-4117.
67. Colodny L, Lynch K, Farber C, Papish S, et al. Results of a study to evaluate the use of Propax to reduce adverse effects of chemotherapy. JANA. 2000;2:17-25.
68. Paradies G, Petrosillo G, Pistolese M, Ruggiero F. Reactive oxygen species affect mitochondrial electron transport complex I activity through oxidative cardiolipin damage. Gene. 2002;286:135-141.
69. Kowald A. The mitochondrial theory of aging: do damaged mitochondria accumulate by delayed degradation? Exp Gerontol. 1999;34:605-612.
70. Koboska J, Coskun P, Esposito L, Wallace DC. Increased mitochondrial oxidative stress in the Sod2(+/-) mouse results in age-related decline of mitochondrial function culminating in increased apoptosis. Proc Nat Acad Sci USA. 2001;98:2278-2283.
71. Fernandes G, Chandrasekar B, Luan X, Troyer DA. Modulation of antioxidant enzymes and programmed cell death by n-3 fatty acids. Lipids. 1996;S9:1-6.
72. Johns DR. Seminars in medicine of Beth Israel Hospital, Boston: mitochondrial DNA and Disease. N Engl J Med. 1995;333:638-44.
.
NT Factor is a nutrient complex that is extracted and prepared using a proprietary process. In addition, nutrients, vitamins and probiotic microorganisms are added to the preparation. It contains the following ingredients:
Glycophospholipids: polyunsaturated phosphatidylcholine, other polyunsaturated phosphatidyl lipids and glycolipids.
Proposed purpose: repair and maintenance of membrane lipids.
Probiotics: Bifido bacterium, Lactobacillus acidophilus, and Lactobacillus bacillus in a freeze-dried, microencapsulated form with appropriate growth nutrients.
Proposed purpose: supports digestion, gut epithelium and the immune system.
Food Supplements, Vitamins, and Growth Medium: bacterial growth factors to support probiotic growth, including defatted rice bran, arginine, beet root fiber extract, blackstrap molasses, glycine, magnesium sulfate, para-amino-benzoate, leek extract, pantethine (bifidus growth factor), taurine, garlic extract, calcium borogluconate, artichoke extract, potassium citrate, calcium sulfate, spirulina, bromelain, natural vitamin E, calcium ascorbate, alpha-lipoic acid, oligosaccharides, vitamin B6, niacinamide, riboflavin, inositol, niacin, calcium pantothenate, thiamin, vitamin B12, folic acid, chromium picolinate.
Proposed purpose: antioxidants support lipids from oxidation, growth medium supports probiotics and gut epithelium, vitamins support general health and the immune system, and food supplements support lipids from enzymatic digestion and oxidation.
Frequently Asked Questions (FAQ’s)
Q. Should OPTI Factor be taken with food?
A. Any products containing NT Factor® may be taken with or without food. Any products containing NT Factor® also utilize NT Factor® as the tablet base. Since NT Factor® contains a wide range of foods and probiotics all the needed coenzymes and cofactors are either present or created as a metabolite.
Q. What separates OPTI Factor™ for other products that claim to fight fatigue?
A. OPTI Factor containing NT Factor™ is the only product proven in clinical trials to reduce and eliminate fatigue. No other product had been proven to provide the same benefit. Some products create the illusion by discussing aspects of the energy producing process or the oxidation and/or transport of pre-energy compounds like sugar and/or fats. However, NT Factor® is the only substance to actually be evaluated successfully for fatigue. Furthermore, pre-clinical trials demonstrated that NT Factor® would also work in animals.
Q. How can OPTI Factor™ improve my health?
A. OPTI Factor containing NT Factor® improves membrane health and all the functions of a membrane. That includes the cell membrane and all membrane enclosed compartments within the cell such as mitochondria. Since the aging process and the development of chronic disease are both accompanied by deteriorating membranes it is clear that OPTI Factor will slow or change this direction?
Q. What are the anti-aging benefits of OPTI Factor™?
A. OPTI Factor containing NT Factor® is better described as a healthy Aging solution. NT Factor® is proven in clinical trials and per-clinical trials to maximize a person’s energy. It is also proven to improve membrane potential and that is the driving force of metabolism. As a result of consuming OPTI Factor regularly a person will get more out of the food they eat and the nutrients (such as multi-vitamin) they consume.
Q. How can OPTI Factor™ improve my quality of life?
A. By improving nutrition, energy, anti-oxidant protection and all membrane mediated functions (an example would be the anti-oxidants produced by the cell itself or the hormones produced and consumed).
Q. How does OPTI Factor™ counteract cellular damage from free radicals?
A. Free Radicals damage us by damaging the fats (called phospholipids) in the membranes of our cells.OPTI Factor simply replaces damaged phospholipids with healthy ones. That is why it is called ‘Lipid Replacement Therapy’.
Q. How can OPTI Factor™ support my immune function?
A. Yes, because OPTI Factor improves the available energy which your immune system needs to function. The easiest way to realize this is to know that when you are tired it is easier to become sick. It also improves the ability to produce the cells that make up your immune system
Q. How does OPTI Factor™ differ from other nutrients that help cellular function?
A.OPTI Factor provides the ability to restore lost membrane function. Typically, nutrients can help to mitigate future damage but only NT Factor® is proven to be able to restore lost membrane function. An example is that we expect older people to have less energy. In one clinical trial NT Factor® restored the energy producing ability (called mitochondrial function) in elderly people to levels normal for young healthy adults.
Q. To what extent has OPTI Factor™ been clinically proven?
A.. Extensively, with pre-clinical, clinical and review studies. Everything is posted for download at on this website.
Q. How many people have taken OPTI Factor® so far?
A.. NT Factor® has been available in excess of ten tears and over a million doses have been consumed with no adverse reactions. OPTI Factor is a new Formula specifically formulated for maximum performance.
Q. How safe is OPTI Factor™?
A. OPTI Factor containing NT Factor® is completely composed of food and food extracts in moderate doses. It does not contain any stimulants, alkaloids, preservative, hormone (or like-hormone compounds) or non-food material whatsoever. It does not contain any common allergens such as wheat, dairy, soy proteins, animal by-products, artificial flavorings, digestible plastics or sugar.
Q. Can children take OPTI Factor™?
A. Yes, in fact children with mitochondrial cytopathies are encouraged to use OPTI Factor as a daily dietary supplement.
Q. What are the precautions when taking OPTI Factor?
A. There are none.
Q. How doe OPTI Factor improve fatigue caused by exercise?
A. By improving mitochondrial function and reducing the damage done to the membranes from exercise. A person experiencing soreness after exercise is an example of the damage to membrane.
Q. What can I expect when I take OPTI Factor™? How long until the effect take place?
A. OPTI Factor containing NT Factor® works by supplying and saturating mucosal membrane with phospholipid and then the phospholipid diffusing throughout the body. This process begins immediately and generally takes between 2 and 3 weeks before the individual begins to really feel the improvement.
Q. Can I take OPTI Factor™ with medications? What should I avoid when taking OPTI Factor™?
A. Yes, OPTI Factor does not interfere with any community standard of care. Consumers are encouraged to discuss consuming OPTI Factor with their doctor and the doctor may obtain any assistance and research by calling 1-800-950-0387.
Q. It is best to take OPTI Factor™ with or without food? What is recommended?
A.OPTI Factor may be taken with or without food. If a meal is very fatty then OPTI Factor should be taken 20 minutes or more before the meal. The reason is that the fat in the meal can interfere with the phospholipids. Meal replacement products that contain lecithin should also be taken twenty minutes after OPTI Factor.
Q. Why is improvement in mitochondrial so important for optimizing my health?
A. Mitochondria does many things one of which is produce energy and process food. Energy is the currency of life. If you have a lot then you are well off. If you don’t then life is difficult.
Q. Can OPTI Factor™ improve nerve function? How?
A. Yes, nerve cells are composed of phospholipids and proteins. The phospholipids determine how well the proteins work and the integrity of the nerve cell. OPTI Factor simply replaces damaged phospholipid with vital healthy phospholipid.
Q. How does OPTI Factor™ work in combination with other supplements such as vitamins, minerals, herbs and foods?
A.OPTI Factor will make other nutrients more effective because OPTI Factor containing NT Factor® is proven to improve membrane potential. That refers to the electrical activity of a membrane and that drives metabolism. A quick example is that the interior of a cell is negatively charged. If you improve the potential you increase the intensity of the negative charge. Calcium, for example is positively charged. When calcium passes by the cell the negative charge pulls in the positively charged mineral. An easy analogy is a vacuum. Improved potential is improved ability to attract nutrition to cross over the cell where it is actually used.
Q. Can OPTI Factor™ improve digestive function?
A.OPTI Factor is particularly beneficial because in addition to improving the health of the cell membranes that line the digestive tract it contains the pre- & probiotics needed to establish healthy microflora (friendly bacteria).
Q. What is the importance of probiotics in OPTI Factor™? Explain.
A. Probiotics are nutrients that make up or cause to make the friendly bacteria in our digestive tracts, mostly the large intestine. This bacteria is important because it is the final stage of digestion and the backbone of our immune system.
Q. How can OPTI Factor™ improve the function of organs of my body?
A. All the parts of our body are made up of cells and require energy. OPTI Factor containing NT Factor® is proven to provide these benefits.
Q. Can OPTI Factor® improve brain function? How? Is there any evidence?
A. Yes, by improving cellular function. The clinical evidence supports this directly and indirectly.
Q. Explain why phospholipids in OPTI Factor are so important in repairing and replacing damaged cell membranes.
A.. Cell membrane phospholipids become dehydrated and stiff as they age. OPTI Factor can simply replace these with healthy vital phospholipids.
Q. What is membrane potential and why is it important for overall health?
A. Membrane potential is the electrical activity or intensity of the cell membrane. It drives metabolism by managing the transport processes of nutrients across the cell membrane.
Q. How does OPTI Factor™ affect membrane potential?
A.OPTI Factor increase membrane potential. OPTI Factor improves the ability of the other nutrients to work.
LABEL FACTS
|
References
1. Kroenke K, Wood DR, Mangelsdorff AD, et al. Chronic fatigue in primary care. Prevalence, patient characteristics, and outcome. JAMA 1988; 260:929-934.
2. Morrison JD. Fatigue as a presenting complaint in family practice. J Family Pract 1980; 10:795-801.
3. McDonald E, David AS, Pelosi AJ, Mann AH. Chronic fatigue in primary care attendees. Psychol Med 1993; 23:987-998.
4. Piper BF, Linsey AM, Dodd MJ. Fatigue mechanism in cancer. Oncol Nursing Forum 1987; 14:17-23.
5. Piper BF, Dribble SL, Dodd MJ, et al. The revised Piper Fatigue Scale: psychometric evaluation in women with breast cancer. Oncol Nursing Forum 1998; 25:667-684.
6. Agadjanyan, M., Vasilevko, V., Ghochikyan, et al. Nutritional supplement (NT Factor) restores mitochondrial function and reduces moderately severe fatigue in aged subjects. J Chronic Fatigue Syndr 2003; 11(4):in press.
7. Ellithorpe RR, Settineri R, Nicolson GL. Pilot Study: Reduction of fatigue by use of a dietary supplement containing glycophospholipids. JANA 2003; 6(1):23-28.
8. Richter C, Par JW, Ames B. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc Nat Acad Sci USA 1998; 85: 6465-6467.
9. Wei YH, Lee HC. Oxidative stress, mitochondrial DNA mutation and impairment of antioxidant enzymes in aging. Exp Biol Med 2002; 227:671-682.
10. Spector AA, Yorek MA. 1985. Membrane Lipid composition and cellular function. J Lipid Res 1985; 26:10105.
11. Harman D. Aging: A theory based on free radical and radiation chemistry. J Gerontol 1956; 2:298-300.
12. Chen D, Cao G, Hastings T et al. Age-dependent decline of DNA repair activity for oxidative lesions in rat brain mitochondria. J Neurochem 2002; 81:1273-1284.
13. Oslewacz HD. Genes, mitochondria and aging in filamentous fungi. Ageing Res Rev 2002; 1:425-442.
14. Barja G. Endogenous oxidative stress: relationship to aging, longevity and caloric restriction. Ageing Res Rev 2002; 1:397-411.
15. Xu D, Finkel T. A role for mitochondria as potential regulators of cellular life span. Biochem Biophysics Res Commun 2002; 294:245-248.
16. Ames BM. Micronutrients prevent cancer and delay aging. Toxicol Lett 1998; 102:1035-1038.
17. De AK, Darad R. Age-associated changes in antioxidants and antioxidative enzymes in rats. Mech Ageing Dev 1991; 59: 123-128.
18. Arivazhagan P, Ramanathan K, Panneerselvam C . Effect of DL-alpha-lipoic acid on mitochondrial enzymes in aged rats. Chem Biol Interact 2001; 138:189-198.
19. Sharman EH, Bondy SC. Effects of age and dietary antioxidants on cerebral electron transport chain activity. Neurobiol Aging 2001; 22(4): 629-634.
20. Sugiyama S, Yamada K, Ozawa T. Preservation of mitochondrial respiratory function by coenzyme Q10 in aged rat skeletal muscle. Biochem Mol Biol Int 1995; 37: 1111-1120.
21. Lin M, Simon D, Ahn C, Lauren K, Beal MF. High aggregrate burden of somatic mtDNA point mutations in aging and Alzheimer’s disease brain. Human Mol Genet 2002; 11:133-145.
22. Sastre J, Pallardo F V, Garcia de la Asuncion J, Vina J. Mitochondria, oxidative stress and aging. Free Radical Res 2000; 32(3): 189-198.
23. Kagan T, Davis C, Lin L, Zakeri Z. Coenzyme Q10 can in some circumstances block apoptosis, and this effect is mediated through mitochondria. Ann NY Acad Sci 1999; 887:31-47.
24. Matthews RT, Yang L, Browne S, et al. Coenzyme Q10 administration increases brain mitochondrial concentrations and exerts neuroprotective effects. Proc Natl Acad Sci USA 1998; 95: 8892-8897.
25. Miquel, J. Can antioxidant diet supplementation protect against age-related mitochondrial damage? Ann NY Acad Sci 2002; 959:317-347.
26. Hu HL, Forsey RJ, Blades TJ, Barratt ME, Parmar P, Powell JR. Antioxidants may contribute in the fight against ageing: an in vitro model. Mech Ageing Dev 2000; 121: 217-230.
27. Passi S, De Pita O, Puddu P, Littarru GP. Lipophilic antioxidants in human sebum and aging. Free Radical Res 2002; 36: 471-477.
28. Forsyth LM, Preuss HG, MacDowell AL, et al. Therapeutic effects of oral NADH on the symptoms of patients with chronic fatigue syndrome. Ann Allergy Asthma Immunol 1999; 82: 185-191.
29. Seidman M, Khan MJ, Tang WX, Quirk WS. Influence of lecithin on mitochondrial DNA and age-related hearing loss. Otolaryngol Head Neck Surg 2002; 127:138-144.
30. Kim MJ, Cooper DD, Hayes SF, Spangrude GJ. Rhodamine-123 staining in hematopoietic stem cells of young mice indicates mitochondrial activation rather than dye efflux. Blood 1998; 91: 4106-4117.
31. Colodny L, Lynch K, Farber C, Papish S, et al. Results of a study to evaluate the use of Propax to reduce adverse effects of chemotherapy. JANA 2000; 2: 17-25.
32. Paradies G, Petrosillo G, Pistolese M, Ruggiero F. Reactive oxygen species affect mitochondrial electron transport complex I activity through oxidative cardiolipin damage. Gene 2002; 286:135-141.
33. Kowald A. The mitochondrial theory of aging: do damaged mitochondria accumulate by delayed degradation? Exp Gerontol 1999; 34:605-612.
34. Koboska J, Coskun P, Esposito L, Wallace DC. Increased mitochondrial oxidative stress in the Sod2(+/-) mouse results in age-related decline of mitochondrial function culminating in increased apoptosis. Proc Nat Acad Sci USA 2001; 98:2278-2283.
35. Johns DR. 1995. Seminars in medicine of Beth Israel Hospital, Boston: Mitochondrial DNA and Disease. N Engl J Med. 1995; 333: 638-44.
Figure 1. Piper Fatigue Scores and mitochondrial function of moderately fatigued subjects (>60 years-old) before, during and after use of NTFactor. Fatigue was determined using the Piper Fatigue Scale. Mitochondrial function was determined by cytofluorographic analysis of 10 mM Rhodamine-123 incorporation into the mitochondrial membranes of blood monocytes isolated from fatigued subjects. *p<0.001 compared to study subjects at time=0.